The roles of NADPH-oxidase and nNOS for the increased oxidative stress and the oxygen consumption in the diabetic kidney
- PMID: 20583310
- PMCID: PMC2897070
- DOI: 10.1002/dmrr.1099
The roles of NADPH-oxidase and nNOS for the increased oxidative stress and the oxygen consumption in the diabetic kidney
Abstract
Background: Sustained hyperglycaemia induces increased renal oxygen consumption resulting in reduced oxygen availability in the diabetic kidney. We investigated the roles of the nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase and the neuronal nitric oxide synthase (nNOS) for the increased oxygen consumption in streptozotocin-diabetic rats.
Methods: Oxygen consumption was measured in isolated proximal tubular cells (PTC) from streptozotocin-induced diabetic rats (n = 7-9 per group) with and without chronic treatment with apocynin, a NADPH-oxidase inhibitor, or S-methyl-L-thiocitrulline (SMTC), a selective nNOS inhibitor, or a combination of the two and the results were compared to normoglycaemic controls (n = 10). Oxidative stress was estimated from thiobarbituric acid reactive substances and protein expression measured by Western blot.
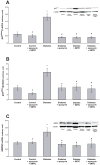
Results: Proximal tubular cells from untreated diabetic rats had increased oxygen consumption compared to controls (40.6 +/- 7.9 versus 10.9 +/- 2.0 nmol/mg protein/min). All treatments reduced the diabetes-induced increase in oxygen consumption (apocynin 10.5 +/- 1.7, SMTC 19.7 +/- 3.0 and apocynin + SMTC 21.6 +/- 3.6 nmol/mg protein/min). Neither apocynin nor SMTC had any effect on the oxygen consumption in cells pre-incubated with ouabain, an inhibitor of active electrolyte transport. Oxidative stress was elevated in the diabetic kidney and inhibited by all treatments. The increased oxygen consumption by diabetic proximal tubular cells correlated with increased protein expressions of p47(phox) and nNOS and the treatments prevented these increases.
Conclusions: Diabetes induces oxidative stress, which increases oxygen consumption in proximal tubular cells. Inhibition of either NADPH-oxidase or nNOS prevented the increased oxygen consumption. The effect of blocking both these enzymes was less than additive suggesting overlapping pathways which warrant further studies.
Copyright (c) 2010 John Wiley & Sons, Ltd.
Figures
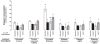

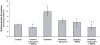
Similar articles
-
Nitric oxide originating from NOS1 controls oxygen utilization and electrolyte transport efficiency in the diabetic kidney.Am J Physiol Renal Physiol. 2010 Feb;298(2):F416-20. doi: 10.1152/ajprenal.00229.2009. Epub 2009 Nov 18. Am J Physiol Renal Physiol. 2010. PMID: 19923416 Free PMC article.
-
NADPH oxidase inhibition reduces tubular sodium transport and improves kidney oxygenation in diabetes.Am J Physiol Regul Integr Comp Physiol. 2012 Jun 15;302(12):R1443-9. doi: 10.1152/ajpregu.00502.2011. Epub 2012 May 2. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22552796 Free PMC article.
-
Apocynin improves erectile function in diabetic rats through regulation of NADPH oxidase expression.J Sex Med. 2012 Dec;9(12):3041-50. doi: 10.1111/j.1743-6109.2012.02960.x. Epub 2012 Oct 22. J Sex Med. 2012. PMID: 23088159
-
Neuronal nitric oxide synthase and human vascular regulation.Trends Cardiovasc Med. 2009 Nov;19(8):256-62. doi: 10.1016/j.tcm.2010.02.007. Trends Cardiovasc Med. 2009. PMID: 20447567 Free PMC article. Review.
-
NADPH oxidases in the kidney.Antioxid Redox Signal. 2006 Sep-Oct;8(9-10):1597-607. doi: 10.1089/ars.2006.8.1597. Antioxid Redox Signal. 2006. PMID: 16987014 Review.
Cited by
-
Redox regulation of stem/progenitor cells and bone marrow niche.Free Radic Biol Med. 2013 Jan;54:26-39. doi: 10.1016/j.freeradbiomed.2012.10.532. Epub 2012 Oct 17. Free Radic Biol Med. 2013. PMID: 23085514 Free PMC article. Review.
-
Endothelin type A receptor inhibition normalises intrarenal hypoxia in rats used as a model of type 1 diabetes by improving oxygen delivery.Diabetologia. 2015 Oct;58(10):2435-42. doi: 10.1007/s00125-015-3690-9. Epub 2015 Jul 15. Diabetologia. 2015. PMID: 26173672
-
Metabolic Rewiring Is Essential for AML Cell Survival to Overcome Autophagy Inhibition by Loss of ATG3.Cancers (Basel). 2021 Dec 6;13(23):6142. doi: 10.3390/cancers13236142. Cancers (Basel). 2021. PMID: 34885250 Free PMC article.
-
Ischemic postconditioning protects the heart against ischemia-reperfusion injury via neuronal nitric oxide synthase in the sarcoplasmic reticulum and mitochondria.Cell Death Dis. 2016 May 12;7(5):e2222. doi: 10.1038/cddis.2016.108. Cell Death Dis. 2016. PMID: 27171264 Free PMC article.
-
Apigenin-Loaded Solid Lipid Nanoparticle Attenuates Diabetic Nephropathy Induced by Streptozotocin Nicotinamide Through Nrf2/HO-1/NF-kB Signalling Pathway.Int J Nanomedicine. 2020 Nov 19;15:9115-9124. doi: 10.2147/IJN.S256494. eCollection 2020. Int J Nanomedicine. 2020. Retraction in: Int J Nanomedicine. 2022 Aug 04;17:3457-3458. doi: 10.2147/IJN.S384666. PMID: 33244230 Free PMC article. Retracted.
References
-
- Korner A, Eklof AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43:629–633. - PubMed
-
- Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46:1153–1160. - PubMed
-
- Tojo A, Asaba K, Onozato ML. Suppressing renal NADPH oxidase to treat diabetic nephropathy. Expert Opin Ther Targets. 2007;11:1011–1018. - PubMed
-
- Asaba K, Tojo A, Onozato ML, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. - PubMed
-
- Satoh M, Fujimoto S, Haruna Y, et al. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F1144–1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

