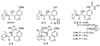
Asymmetric vinylogous aldol reaction of silyloxy furans with a chiral organic salt
- PMID: 20583835
- PMCID: PMC2925177
- DOI: 10.1021/ja103331t
Asymmetric vinylogous aldol reaction of silyloxy furans with a chiral organic salt
Abstract
Despite their synthetic significance there is a general lack of asymmetric vinylogous aldol reactions that tolerate variations of both the silyloxy furans and aldehydes. We have developed a new chiral organic catalyst based on a carboxylate-ammonium salt prepared from a thiourea-amine and a carboxylic acid. This new catalyst enabled us to develop an efficient asymmetric vinylogous aldol reaction of unprecedented scope with respect to both 2-trimethylsilyloxy furans and aldehydes.
Figures
Similar articles
-
Organocatalytic asymmetric direct vinylogous aldol reactions of γ-crotonolactone with aromatic aldehydes.Chem Commun (Camb). 2011 Jan 21;47(3):1027-9. doi: 10.1039/c0cc04191b. Epub 2010 Nov 10. Chem Commun (Camb). 2011. PMID: 21069217
-
Asymmetric synthesis of beta-hydroxy amino acids via aldol reactions catalyzed by chiral ammonium salts.J Org Chem. 2004 Sep 17;69(19):6489-92. doi: 10.1021/jo049283u. J Org Chem. 2004. PMID: 15357616
-
General switch in regioselectivity in the Mukaiyama aldol reaction of silyloxyfuran with aldehydes in aqueous solvents.Chem Commun (Camb). 2012 Nov 18;48(89):11029-31. doi: 10.1039/c2cc36656h. Chem Commun (Camb). 2012. PMID: 23037879
-
[Development of Green Asymmetric Organocatalytic Synthesis].Yakugaku Zasshi. 2021;141(10):1137-1145. doi: 10.1248/yakushi.21-00130. Yakugaku Zasshi. 2021. PMID: 34602510 Review. Japanese.
-
Current progress in the asymmetric aldol addition reaction.Chem Soc Rev. 2004 Feb 20;33(2):65-75. doi: 10.1039/b202901d. Epub 2004 Jan 20. Chem Soc Rev. 2004. PMID: 14767502 Review.
Cited by
-
Silyldienolates in Organocatalytic Enantioselective Vinylogous Mukaiyama-Type Reactions: A Review.Molecules. 2021 Nov 16;26(22):6902. doi: 10.3390/molecules26226902. Molecules. 2021. PMID: 34833996 Free PMC article. Review.
-
Carboxylate Catalysis: A Catalytic O-Silylative Aldol Reaction of Aldehydes and Ethyl Diazoacetate.J Org Chem. 2023 Oct 20;88(20):14396-14403. doi: 10.1021/acs.joc.3c01304. Epub 2023 Sep 28. J Org Chem. 2023. PMID: 37768196 Free PMC article.
-
Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones.Chem Rev. 2017 Aug 9;117(15):10502-10566. doi: 10.1021/acs.chemrev.7b00151. Epub 2017 Jun 22. Chem Rev. 2017. PMID: 28640622 Free PMC article. Review.
-
Iridium-catalyzed regioselective and enantioselective allylation of trimethylsiloxyfuran.J Am Chem Soc. 2012 Sep 19;134(37):15249-52. doi: 10.1021/ja306850b. Epub 2012 Sep 11. J Am Chem Soc. 2012. PMID: 22954355 Free PMC article.
-
Combined C-H functionalization/Cope rearrangement with vinyl ethers as a surrogate for the vinylogous Mukaiyama aldol reaction.J Am Chem Soc. 2011 Aug 10;133(31):11940-3. doi: 10.1021/ja2051155. Epub 2011 Jul 18. J Am Chem Soc. 2011. PMID: 21739977 Free PMC article.
References
-
- Carmen Zafra-Polo M, Figadère B, Gallardo T, Tormo J, Cortes D. Phytochemistry. 1998;48:1087.
-
-
For reviews of vinylogous aldol reactions, see: Casiraghi G, Zanardi F, Appendino G, Rassu G. Chem. Rev. 2000;100:1929. Denmark SE, Jr, J.R.H, Beutner GL. Angew. Chem. Int. Ed. 2005;44:4682. Kalesse M. Top. Curr. Chem. 2005;244:43.
-
-
-
For enantioselective vinylogous aldol reactions of 2-silyloxyfurans, see: Szlosek M, Franck X, Figadere B, Cave A. J. Org. Chem. 1998;63:5169. Evans DA, Kozlowski MC, Murry JA, Burgey CS, Campos KR, Connell BT, Staples RJ. J. Am. Chem. Soc. 1999;121:669. Szlosek M, Figadère B. Angew. Chem. Int. Ed. 2000;39:1799. Matsuoka Y, Irie R, Katsuki T. Chem. Lett. 2003;32:584. Onitsuka S, Matsuoka Y, Irie R, Katsuki T. Chem. Lett. 2003;32:974. Nagao H, Yamane Y, Mukaiyama T. Chem. Lett. 2007;36:8. Palombi L, Acocella MR, Celenta N, Massa A, Villano R, Scettri A. Tetrahedron Asymmetry. 2006;17:3300. Sedelmeier J, Hammerer T, Bolm C. Org. Lett. 2008;10:917.
-
-
- Ube H, Shimada N, Terada M. Angew. Chem. Int. Ed. 2010;49:1858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources