Early fluid resuscitation with hyperoncotic hydroxyethyl starch 200/0.5 (10%) in severe burn injury
- PMID: 20584291
- PMCID: PMC2911771
- DOI: 10.1186/cc9086
Early fluid resuscitation with hyperoncotic hydroxyethyl starch 200/0.5 (10%) in severe burn injury
Abstract
Introduction: Despite large experience in the management of severe burn injury, there are still controversies regarding the best type of fluid resuscitation, especially during the first 24 hours after the trauma. Therefore, our study addressed the question whether hyperoncotic hydroxyethyl starch (HES) 200/0.5 (10%) administered in combination with crystalloids within the first 24 hours after injury is as effective as 'crystalloids only' in severe burn injury patients.
Methods: 30 consecutive patients were enrolled to this prospective interventional open label study and assigned either to a traditional 'crystalloids only' or to a 'HES 200/0.5 (10%)' volume resuscitation protocol. Total amount of fluid administration, complications such as pulmonary failure, abdominal compartment syndrome, sepsis, renal failure and overall mortality were assessed. Cox proportional hazard regression analysis was performed for binary outcomes and adjustment for potential confounders was done in the multivariate regression models. For continuous outcome parameters multiple linear regression analysis was used.
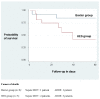
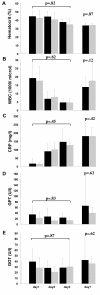
Results: Group differences between patients receiving crystalloids only or HES 200/0.5 (10%) were not statistically significant. However, a large effect towards increased overall mortality (adjusted hazard ratio 7.12; P = 0.16) in the HES 200/0.5 (10%) group as compared to the crystalloids only group (43.8% versus 14.3%) was present. Similarly, the incidence of renal failure was 25.0% in the HES 200/0.5 (10%) group versus 7.1% in the crystalloid only group (adjusted hazard ratio 6.16; P = 0.42).
Conclusions: This small study indicates that the application of hyperoncotic HES 200/0.5 (10%) within the first 24 hours after severe burn injury may be associated with fatal outcome and should therefore be used with caution.
Trial registration: NCT01120730.
Figures
References
-
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. - DOI - PubMed
-
- Reinhart K, Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Deufel T, Hartog C, Gerlach H, Stuber F, Volk HD, Quintel M, Loeffler M. [Study protocol of the VISEP study: Response of the SepNet study group] Anaesthesist. 2008;57:723–728. doi: 10.1007/s00101-008-1391-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

