Lipoprotein(a) accelerates atherosclerosis in uremic mice
- PMID: 20584868
- PMCID: PMC2936745
- DOI: 10.1194/jlr.M006742
Lipoprotein(a) accelerates atherosclerosis in uremic mice
Abstract
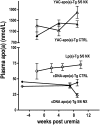
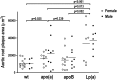
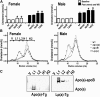
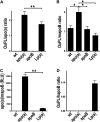
Uremic patients have increased plasma lipoprotein(a) [Lp(a)] levels and elevated risk of cardiovascular disease. Lp(a) is a subfraction of LDL, where apolipoprotein(a) [apo(a)] is disulfide bound to apolipoprotein B-100 (apoB). Lp(a) binds oxidized phospholipids (OxPL), and uremia increases lipoprotein-associated OxPL. Thus, Lp(a) may be particularly atherogenic in a uremic setting. We therefore investigated whether transgenic (Tg) expression of human Lp(a) increases atherosclerosis in uremic mice. Moderate uremia was induced by 5/6 nephrectomy (NX) in Tg mice with expression of human apo(a) (n = 19), human apoB-100 (n = 20), or human apo(a) + human apoB [Lp(a)] (n = 15), and in wild-type (WT) controls (n = 21). The uremic mice received a high-fat diet, and aortic atherosclerosis was examined 35 weeks later. LDL-cholesterol was increased in apoB-Tg and Lp(a)-Tg mice, but it was normal in apo(a)-Tg and WT mice. Uremia did not result in increased plasma apo(a) or Lp(a). Mean atherosclerotic plaque area in the aortic root was increased 1.8-fold in apo(a)-Tg (P = 0.025) and 3.3-fold (P = 0.0001) in Lp(a)-Tg mice compared with WT mice. Plasma OxPL, as detected with the E06 antibody, was associated with both apo(a) and Lp(a). In conclusion, expression of apo(a) or Lp(a) increased uremia-induced atherosclerosis. Binding of OxPL on apo(a) and Lp(a) may contribute to the atherogenicity of Lp(a) in uremia.
Figures

Similar articles
-
Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice.Circulation. 2008 Aug 12;118(7):743-53. doi: 10.1161/CIRCULATIONAHA.108.786822. Epub 2008 Jul 28. Circulation. 2008. PMID: 18663084
-
High levels of lipoprotein(a) in transgenic mice exacerbate atherosclerosis and promote vulnerable plaque features in a sex-specific manner.Atherosclerosis. 2023 Nov;384:117150. doi: 10.1016/j.atherosclerosis.2023.05.019. Epub 2023 May 30. Atherosclerosis. 2023. PMID: 37290980
-
Lipoprotein(A) with An Intact Lysine Binding Site Protects the Retina From an Age-Related Macular Degeneration Phenotype in Mice (An American Ophthalmological Society Thesis).Trans Am Ophthalmol Soc. 2015;113:T5. Trans Am Ophthalmol Soc. 2015. PMID: 26538774 Free PMC article.
-
Experimental Animal Models Evaluating the Causal Role of Lipoprotein(a) in Atherosclerosis and Aortic Stenosis.Cardiovasc Drugs Ther. 2016 Feb;30(1):75-85. doi: 10.1007/s10557-015-6634-1. Cardiovasc Drugs Ther. 2016. PMID: 26780907 Review.
-
Oxidized phospholipid modification of lipoprotein(a): Epidemiology, biochemistry and pathophysiology.Atherosclerosis. 2022 May;349:92-100. doi: 10.1016/j.atherosclerosis.2022.04.001. Atherosclerosis. 2022. PMID: 35606081 Review.
Cited by
-
Mouse plasminogen has oxidized phosphatidylcholine adducts that are not metabolized by lipoprotein-associated phospholipase A₂under basal conditions.Int J Mol Sci. 2010;11(12):5339-47. doi: 10.3390/ijms11125339. Epub 2010 Dec 22. Int J Mol Sci. 2010. PMID: 21614211 Free PMC article.
-
Lipoprotein(a) in nephrological patients.Clin Res Cardiol Suppl. 2017 Mar;12(Suppl 1):27-30. doi: 10.1007/s11789-017-0086-z. Clin Res Cardiol Suppl. 2017. PMID: 28181057 Free PMC article. Review.
-
Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease?J Lipid Res. 2016 May;57(5):745-57. doi: 10.1194/jlr.R060582. Epub 2015 Dec 8. J Lipid Res. 2016. PMID: 26647358 Free PMC article. Review.
-
Modified Lipids and Lipoproteins in Chronic Kidney Disease: A New Class of Uremic Toxins.Toxins (Basel). 2016 Dec 16;8(12):376. doi: 10.3390/toxins8120376. Toxins (Basel). 2016. PMID: 27999257 Free PMC article. Review.
-
FGF21 inhibits apolipoprotein(a) expression in HepG2 cells via the FGFR1-ERK1/2-Elk-1 pathway.Mol Cell Biochem. 2014 Aug;393(1-2):33-42. doi: 10.1007/s11010-014-2044-0. Epub 2014 Apr 4. Mol Cell Biochem. 2014. PMID: 24700140
References
-
- Go A. S., Chertow G. M., Fan D., McCulloch C. E., Hsu C. Y. 2004. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351: 1296–1305. - PubMed
-
- Fellstrom B. C., Jardine A. G., Schmieder R. E., Holdaas H., Bannister K., Beutler J., Chae D. W., Chevaile A., Cobbe S. M., Gronhagen-Riska C., et al. 2009. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360: 1395–1407. - PubMed
-
- Wanner C., Krane V., Marz W., Olschewski M., Mann J. F., Ruf G., Ritz E. 2005. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353: 238–248. - PubMed
-
- Massy Z. A., Ivanovski O., Nguyen-Khoa T., Angulo J., Szumilak D., Mothu N., Phan O., Daudon M., Lacour B., Drueke T. B., et al. 2005. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J. Am. Soc. Nephrol. 16: 109–116. - PubMed
-
- Bro S., Bentzon J. F., Falk E., Andersen C. B., Olgaard K., Nielsen L. B. 2003. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J. Am. Soc. Nephrol. 14: 2466–2474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

