Bcl-2 and Bax interact via the BH1-3 groove-BH3 motif interface and a novel interface involving the BH4 motif
- PMID: 20584903
- PMCID: PMC2937903
- DOI: 10.1074/jbc.M110.148361
Bcl-2 and Bax interact via the BH1-3 groove-BH3 motif interface and a novel interface involving the BH4 motif
Abstract
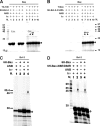
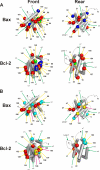
The interaction of Bcl-2 family proteins at the mitochondrial outer membrane controls membrane permeability and thereby the apoptotic program. The anti-apoptotic protein Bcl-2 binds to the pro-apoptotic protein Bax to prevent Bax homo-oligomerization required for membrane permeabilization. Here, we used site-specific photocross-linking to map the surfaces of Bax and Bcl-2 that interact in the hetero-complex formed in a Triton X-100 micelle as a membrane surrogate. Heterodimer-specific photoadducts were detected from multiple sites in Bax and Bcl-2. Many of the interaction sites are located in the Bcl-2 homology 3 (BH3) region of Bax and the BH1-3 groove of Bcl-2 that likely form the BH3-BH1-3 groove interface. However, other interaction sites form a second interface that includes helix 6 of Bax and the BH4 region of Bcl-2. Loss-of-function mutations in the BH3 region of Bax and the BH1 region of Bcl-2 disrupted the BH3-BH1-3 interface, as expected. Surprisingly the second interface was also disrupted by these mutations. Similarly, a loss-of-function mutation in the BH4 region of Bcl-2 that forms part of the second interface also disrupted both interfaces. As expected, both kinds of mutation abolished Bcl-2-mediated inhibition of Bax oligomerization in detergent micelles. Therefore, Bcl-2 binds Bax through two interdependent interfaces to inhibit the pro-apoptotic oligomerization of Bax.
Figures




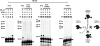


Similar articles
-
After embedding in membranes antiapoptotic Bcl-XL protein binds both Bcl-2 homology region 3 and helix 1 of proapoptotic Bax protein to inhibit apoptotic mitochondrial permeabilization.J Biol Chem. 2014 Apr 25;289(17):11873-11896. doi: 10.1074/jbc.M114.552562. Epub 2014 Mar 10. J Biol Chem. 2014. PMID: 24616095 Free PMC article.
-
Bax forms an oligomer via separate, yet interdependent, surfaces.J Biol Chem. 2010 Jun 4;285(23):17614-27. doi: 10.1074/jbc.M110.113456. Epub 2010 Apr 9. J Biol Chem. 2010. PMID: 20382739 Free PMC article.
-
Inhibition of Pro-apoptotic BAX by a noncanonical interaction mechanism.Mol Cell. 2015 Mar 5;57(5):873-886. doi: 10.1016/j.molcel.2015.01.014. Epub 2015 Feb 12. Mol Cell. 2015. PMID: 25684204 Free PMC article.
-
Structure-function analysis of Bcl-2 family proteins. Regulators of programmed cell death.Adv Exp Med Biol. 1996;406:99-112. Adv Exp Med Biol. 1996. PMID: 8910675 Review.
-
Structural biology of the Bcl-2 family of proteins.Biochim Biophys Acta. 2004 Mar 1;1644(2-3):83-94. doi: 10.1016/j.bbamcr.2003.08.012. Biochim Biophys Acta. 2004. PMID: 14996493 Review.
Cited by
-
Hugl-1 induces apoptosis in esophageal carcinoma cells both in vitro and in vivo.World J Gastroenterol. 2013 Jul 14;19(26):4127-36. doi: 10.3748/wjg.v19.i26.4127. World J Gastroenterol. 2013. PMID: 23864775 Free PMC article.
-
Bcl-2-Protein Family as Modulators of IP3 Receptors and Other Organellar Ca2+ Channels.Cold Spring Harb Perspect Biol. 2020 Apr 1;12(4):a035089. doi: 10.1101/cshperspect.a035089. Cold Spring Harb Perspect Biol. 2020. PMID: 31501195 Free PMC article. Review.
-
The C-terminal sequences of Bcl-2 family proteins mediate interactions that regulate cell death.Biochem J. 2024 Jul 17;481(14):903-922. doi: 10.1042/BCJ20210352. Biochem J. 2024. PMID: 38985308 Free PMC article. Review.
-
An Integrated Bioinformatics and Computational Biology Approach Identifies New BH3-Only Protein Candidates.Open Biol J. 2012 May 4;5:6-16. doi: 10.2174/1874196701205010006. Open Biol J. 2012. PMID: 22754595 Free PMC article.
-
Direct interaction of Bax and Bak proteins with Bcl-2 homology domain 3 (BH3)-only proteins in living cells revealed by fluorescence complementation.J Biol Chem. 2013 Feb 15;288(7):4935-46. doi: 10.1074/jbc.M112.422204. Epub 2013 Jan 2. J Biol Chem. 2013. PMID: 23283967 Free PMC article.
References
-
- Youle R. J., Strasser A. (2008) Nat. Rev. Mol. Cell Biol. 9, 47–59 - PubMed
-
- Kvansakul M., Yang H., Fairlie W. D., Czabotar P. E., Fischer S. F., Perugini M. A., Huang D. C., Colman P. M. (2008) Cell Death Differ. 15, 1564–1571 - PubMed
-
- Suzuki M., Youle R. J., Tjandra N. (2000) Cell 103, 645–654 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

