Identification of the chromophores involved in aggregation-dependent energy quenching of the monomeric photosystem II antenna protein Lhcb5
- PMID: 20584907
- PMCID: PMC2934695
- DOI: 10.1074/jbc.M110.124115
Identification of the chromophores involved in aggregation-dependent energy quenching of the monomeric photosystem II antenna protein Lhcb5
Abstract
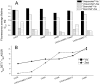
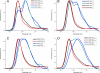
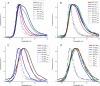
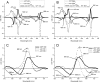
Non-photochemical quenching (NPQ) of excess absorbed light energy is a fundamental process that regulates photosynthetic light harvesting in higher plants. Among several proposed NPQ mechanisms, aggregation-dependent quenching (ADQ) and charge transfer quenching have received the most attention. In vitro spectroscopic features of both mechanisms correlate with very similar signals detected in more intact systems and in vivo, where full NPQ can be observed. A major difference between the models is the proposed quenching site, which is predominantly the major trimeric light-harvesting complex II in ADQ and exclusively monomeric Lhcb proteins in charge transfer quenching. Here, we studied ADQ in both monomeric and trimeric Lhcb proteins, investigating the activities of each antenna subunit and their dependence on zeaxanthin, a major modulator of NPQ in vivo. We found that monomeric Lhcb proteins undergo stronger quenching than light-harvesting complex II during aggregation and that this is enhanced by binding to zeaxanthin, as occurs during NPQ in vivo. Finally, the analysis of Lhcb5 mutants showed that chlorophyll 612 and 613, in close contact with lutein bound at site L1, are important facilitators of ADQ.
Figures

Similar articles
-
A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26.Plant Cell. 2005 Apr;17(4):1217-32. doi: 10.1105/tpc.104.030601. Epub 2005 Mar 4. Plant Cell. 2005. PMID: 15749754 Free PMC article.
-
On the PsbS-induced quenching in the plant major light-harvesting complex LHCII studied in proteoliposomes.Photosynth Res. 2020 May;144(2):195-208. doi: 10.1007/s11120-020-00740-z. Epub 2020 Apr 7. Photosynth Res. 2020. PMID: 32266611
-
Occupancy and functional architecture of the pigment binding sites of photosystem II antenna complex Lhcb5.J Biol Chem. 2009 Mar 20;284(12):8103-13. doi: 10.1074/jbc.M808326200. Epub 2009 Jan 7. J Biol Chem. 2009. PMID: 19129188 Free PMC article.
-
Crystallisation, structure and function of plant light-harvesting Complex II.Biochim Biophys Acta. 2009 Jun;1787(6):753-72. doi: 10.1016/j.bbabio.2009.03.012. Epub 2009 Mar 25. Biochim Biophys Acta. 2009. PMID: 19327340 Review.
-
Energy-Dependent Non-Photochemical Quenching: PsbS, LhcSR, and Other Players.Biochemistry (Mosc). 2025 Jan;90(1):44-60. doi: 10.1134/S000629792460371X. Biochemistry (Mosc). 2025. PMID: 40058973 Review.
Cited by
-
Functional modulation of LHCSR1 protein from Physcomitrella patens by zeaxanthin binding and low pH.Sci Rep. 2017 Sep 11;7(1):11158. doi: 10.1038/s41598-017-11101-7. Sci Rep. 2017. PMID: 28894198 Free PMC article.
-
Multimeric and monomeric photosystem II supercomplexes represent structural adaptations to low- and high-light conditions.J Biol Chem. 2020 Oct 23;295(43):14537-14545. doi: 10.1074/jbc.RA120.014198. Epub 2020 Jun 19. J Biol Chem. 2020. PMID: 32561642 Free PMC article.
-
Identification of pH-sensing Sites in the Light Harvesting Complex Stress-related 3 Protein Essential for Triggering Non-photochemical Quenching in Chlamydomonas reinhardtii.J Biol Chem. 2016 Apr 1;291(14):7334-46. doi: 10.1074/jbc.M115.704601. Epub 2016 Jan 27. J Biol Chem. 2016. PMID: 26817847 Free PMC article.
-
Light-harvesting regulation from leaf to molecule with the emphasis on rapid changes in antenna size.Photosynth Res. 2015 May;124(2):137-58. doi: 10.1007/s11120-015-0115-z. Epub 2015 Mar 14. Photosynth Res. 2015. PMID: 25773873 Review.
-
Acclimation- and mutation-induced enhancement of PsbS levels affects the kinetics of non-photochemical quenching in Arabidopsis thaliana.Planta. 2011 Jun;233(6):1253-64. doi: 10.1007/s00425-011-1380-5. Epub 2011 Feb 22. Planta. 2011. PMID: 21340700
References
-
- Liu Z., Yan H., Wang K., Kuang T., Zhang J., Gui L., An X., Chang W. (2004) Nature 428, 287–292 - PubMed
-
- Kühlbrandt W., Wang D. N., Fujiyoshi Y. (1994) Nature 367, 614–621 - PubMed
-
- Jansson S. (1999) Trends Plant Sci. 4, 236–240 - PubMed
-
- Amunts A., Drory O., Nelson N. (2007) Nature 447, 58–63 - PubMed
-
- Ben-Shem A., Frolow F., Nelson N. (2003) Nature 426, 630–635 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

