Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II
- PMID: 20584908
- PMCID: PMC2934654
- DOI: 10.1074/jbc.M110.133066
Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II
Abstract
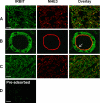
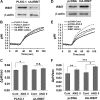
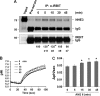
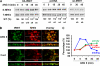
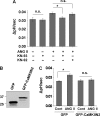
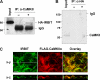
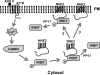
Angiotensin II (ANG II) stimulates renal tubular reabsorption of NaCl by targeting Na(+)/H(+) exchanger NHE3. We have shown previously that inositol 1,4,5-triphosphate receptor-binding protein released with inositol 1,4,5-triphosphate (IRBIT) plays a critical role in stimulation of NHE3 in response to elevated intracellular Ca(2+) concentration ([Ca(2+)](i)). In this study, we investigated the role of IRBIT in mediating NHE3 activation by ANG II. IRBIT is abundantly expressed in the proximal tubules where NHE3 is located. ANG II at physiological concentrations stimulates NHE3 transport activity in a model proximal tubule cell line. ANG II-induced activation of NHE3 was abrogated by knockdown of IRBIT, whereas overexpression of IRBIT enhanced the effect of ANG II on NHE3. ANG II transiently increased binding of IRBIT to NHE3 at 5 min but became dissociated by 45 min. In comparison, it took at least 15 min of ANG II treatment for an increase in NHE3 activity and NHE3 surface expression. The stimulation of NHE3 by ANG II was dependent on changes in [Ca(2+)](i) and Ca(2+)/calmodulin-dependent protein kinases II. Inhibition of CaMKII completely blocked the ANG II-induced binding of IRBIT to NHE3 and the increase in NHE3 surface abundance. Several serine residues of IRBIT are thought to be important for IRBIT binding. Mutations of Ser-68, Ser-71, and Ser-74 of IRBIT decreased binding of IRBIT to NHE3 and its effect on NHE3 activity. In conclusion, our current findings demonstrate that IRBIT is critically involved in mediating activation of NHE3 by ANG II via a Ca(2+)/calmodulin-dependent protein kinases II-dependent pathway.
Figures


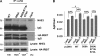
Similar articles
-
IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium.J Biol Chem. 2008 Nov 28;283(48):33544-53. doi: 10.1074/jbc.M805534200. Epub 2008 Sep 30. J Biol Chem. 2008. PMID: 18829453 Free PMC article.
-
The NHERF1 PDZ1 domain and IRBIT interact and mediate the activation of Na+/H+ exchanger 3 by ANG II.Am J Physiol Renal Physiol. 2016 Aug 1;311(2):F343-51. doi: 10.1152/ajprenal.00247.2016. Epub 2016 Jun 8. Am J Physiol Renal Physiol. 2016. PMID: 27279487 Free PMC article.
-
Novel signaling mechanisms of intracellular angiotensin II-induced NHE3 expression and activation in mouse proximal tubule cells.Am J Physiol Renal Physiol. 2012 Dec 15;303(12):F1617-28. doi: 10.1152/ajprenal.00219.2012. Epub 2012 Oct 3. Am J Physiol Renal Physiol. 2012. PMID: 23034941 Free PMC article.
-
IRBIT: a regulator of ion channels and ion transporters.Biochim Biophys Acta. 2014 Oct;1843(10):2195-204. doi: 10.1016/j.bbamcr.2014.01.031. Epub 2014 Feb 8. Biochim Biophys Acta. 2014. PMID: 24518248 Review.
-
Genetic and genomic evidence for an important role of the Na+/H+ exchanger 3 in blood pressure regulation and angiotensin II-induced hypertension.Physiol Genomics. 2019 Apr 1;51(4):97-108. doi: 10.1152/physiolgenomics.00122.2018. Epub 2019 Mar 8. Physiol Genomics. 2019. PMID: 30849009 Free PMC article. Review.
Cited by
-
Ubiquitin-specific peptidase 7 (USP7) and USP10 mediate deubiquitination of human NHE3 regulating its expression and activity.FASEB J. 2020 Dec;34(12):16476-16488. doi: 10.1096/fj.202001875R. Epub 2020 Oct 23. FASEB J. 2020. PMID: 33095475 Free PMC article.
-
The Na+/H+ Exchanger 3 in the Intestines and the Proximal Tubule of the Kidney: Localization, Physiological Function, and Key Roles in Angiotensin II-Induced Hypertension.Front Physiol. 2022 Apr 19;13:861659. doi: 10.3389/fphys.2022.861659. eCollection 2022. Front Physiol. 2022. PMID: 35514347 Free PMC article. Review.
-
Crosstalk between Sodium-Glucose Cotransporter Inhibitors and Sodium-Hydrogen Exchanger 1 and 3 in Cardiometabolic Diseases.Int J Mol Sci. 2021 Nov 24;22(23):12677. doi: 10.3390/ijms222312677. Int J Mol Sci. 2021. PMID: 34884494 Free PMC article. Review.
-
Renal Collectrin Protects against Salt-Sensitive Hypertension and Is Downregulated by Angiotensin II.J Am Soc Nephrol. 2017 Jun;28(6):1826-1837. doi: 10.1681/ASN.2016060675. Epub 2017 Jan 6. J Am Soc Nephrol. 2017. PMID: 28062568 Free PMC article.
-
Splicing variation of Long-IRBIT determines the target selectivity of IRBIT family proteins.Proc Natl Acad Sci U S A. 2017 Apr 11;114(15):3921-3926. doi: 10.1073/pnas.1618514114. Epub 2017 Mar 27. Proc Natl Acad Sci U S A. 2017. PMID: 28348216 Free PMC article.
References
-
- Biemesderfer D., Pizzonia J., Abu-Alfa A., Exner M., Reilly R., Igarashi P., Aronson P. S. (1993) Am. J. Physiol. 265, F736–F742 - PubMed
-
- Aronson P. S. (1996) Kidney Int. 49, 1665–1670 - PubMed
-
- Schultheis P. J., Clarke L. L., Meneton P., Miller M. L., Soleimani M., Gawenis L. R., Riddle T. M., Duffy J. J., Doetschman T., Wang T., Giebisch G., Aronson P. S., Lorenz J. N., Shull G. E. (1998) Nat. Genet. 19, 282–285 - PubMed
-
- Brown G. P., Douglas J. G. (1982) Endocrinology 111, 1830–1836 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

