Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner
- PMID: 20584916
- PMCID: PMC2894457
- DOI: 10.1083/jcb.201001149
Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner
Erratum in
- J Cell Biol. 2010 Nov 15;191(4):891
Abstract
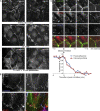
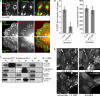
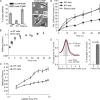
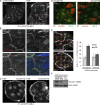
Cell surface receptors integrate chemical and mechanical cues to regulate a wide range of biological processes. Integrin complexes are the mechanotransducers between the extracellular matrix and the actomyosin cytoskeleton. By analogy, cadherin complexes may function as mechanosensors at cell-cell junctions, but this capacity of cadherins has not been directly demonstrated. Furthermore, the molecular composition of the link between E-cadherin and actin, which is needed to sustain such a function, is unresolved. In this study, we describe nanomechanical measurements demonstrating that E-cadherin complexes are functional mechanosensors that transmit force between F-actin and E-cadherin. Imaging experiments reveal that intercellular forces coincide with vinculin accumulation at actin-anchored cadherin adhesions, and nanomechanical measurements show that vinculin potentiates the E-cadherin mechanosensory response. These investigations directly demonstrate the mechanosensory capacity of the E-cadherin complex and identify a novel function for vinculin at cell-cell junctions. These findings have implications for barrier function, morphogenesis, cell migration, and invasion and may extend to all soft tissues in which classical cadherins regulate cell-cell adhesion.
Figures

Comment in
-
Neighborly relations: cadherins and mechanotransduction.J Cell Biol. 2010 Jun 28;189(7):1075-7. doi: 10.1083/jcb.201005151. J Cell Biol. 2010. PMID: 20584914 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

