Sleep restriction for 1 week reduces insulin sensitivity in healthy men
- PMID: 20585000
- PMCID: PMC2927933
- DOI: 10.2337/db09-0699
Sleep restriction for 1 week reduces insulin sensitivity in healthy men
Abstract
Objective: Short sleep duration is associated with impaired glucose tolerance and an increased risk of diabetes. The effects of sleep restriction on insulin sensitivity have not been established. This study tests the hypothesis that decreasing nighttime sleep duration reduces insulin sensitivity and assesses the effects of a drug, modafinil, that increases alertness during wakefulness.
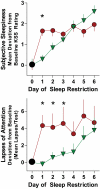
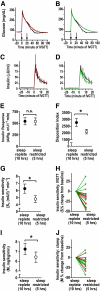
Research design and methods: This 12-day inpatient General Clinical Research Center study included 20 healthy men (age 20-35 years and BMI 20-30 kg/m(2)). Subjects spent 10 h/night in bed for >or=8 nights including three inpatient nights (sleep-replete condition), followed by 5 h/night in bed for 7 nights (sleep-restricted condition). Subjects received 300 mg/day modafinil or placebo during sleep restriction. Diet and activity were controlled. On the last 2 days of each condition, we assessed glucose metabolism by intravenous glucose tolerance test (IVGTT) and euglycemic-hyperinsulinemic clamp. Salivary cortisol, 24-h urinary catecholamines, and neurobehavioral performance were measured.
Results: IVGTT-derived insulin sensitivity was reduced by (means +/- SD) 20 +/- 24% after sleep restriction (P = 0.001), without significant alterations in the insulin secretory response. Similarly, insulin sensitivity assessed by clamp was reduced by 11 +/- 5.5% (P < 0.04) after sleep restriction. Glucose tolerance and the disposition index were reduced by sleep restriction. These outcomes were not affected by modafinil treatment. Changes in insulin sensitivity did not correlate with changes in salivary cortisol (increase of 51 +/- 8% with sleep restriction, P < 0.02), urinary catecholamines, or slow wave sleep.
Conclusions: Sleep restriction (5 h/night) for 1 week significantly reduces insulin sensitivity, raising concerns about effects of chronic insufficient sleep on disease processes associated with insulin resistance.
Trial registration: ClinicalTrials.gov NCT00895570.
Figures


References
-
- Hale L, Peppard PE, Young T. Does the demography of sleep contribute to health disparities? In Sleep Disorders, Their Impact on Public Health. Leger D, Pandi-Perumal SR. Eds. London, Informa Healthcare, 2007, p. 1–17
-
- Cizza G, Skarulis M, Mignot E. A link between short sleep and obesity: building the evidence for causation. Sleep 2005;28:1217–1220 - PubMed
-
- Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 2005;28:1289–1296 - PubMed
-
- Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rossler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep 2004;27:661–666 - PubMed

