Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus
- PMID: 20585035
- PMCID: PMC4457446
- DOI: 10.4049/jimmunol.1000091
Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus
Abstract
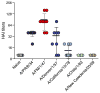
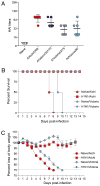
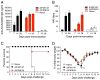
The 2009 H1N1 influenza virus outbreak is the first pandemic of the twenty-first century. Epidemiological data reveal that of all the people afflicted with H1N1 virus, <5% are over 51 y of age. Interestingly, in the uninfected population, 33% of those >60 y old have pre-existing neutralizing Abs against the 2009 H1N1 virus. This finding suggests that influenza strains that circulated 50-60 y ago might provide cross-protection against the swine-origin 2009 H1N1 influenza virus. To test this, we determined the ability of representative H1N1 influenza viruses that circulated in the human population from 1930 to 2000, to induce cross-reactivity to and cross-protection against the pandemic swine-origin H1N1 virus, A/California/04/09. We show that exposure of mice to the 1947 virus, A/FM/1/47, or the 1934 virus, A/PR/8/34, induced robust cross-protective immune responses and these mice were protected against a lethal challenge with mouse-adapted A/California/04/09 H1N1 virus. Conversely, we observed that mice exposed to the 2009 H1N1 virus were protected against a lethal challenge with mouse-adapted 1947 or 1934 H1N1 viruses. In addition, exposure to the 2009 H1N1 virus induced broad cross-reactivity against H1N1 as well as H3N2 influenza viruses. Finally, we show that vaccination with the older H1N1 viruses, particularly A/FM/1/47, confers protective immunity against the 2009 pandemic H1N1 virus. Taken together, our data provide an explanation for the decreased susceptibility of the elderly to the 2009 H1N1 outbreak and demonstrate that vaccination with the pre-1950 influenza strains can cross-protect against the pandemic swine-origin 2009 H1N1 influenza virus.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




References
-
- Gottschalk A, Graham ER. 6-alpha-D-Sialyl-N-acetyl-galactosamine: the neuraminidase-susceptible prosthetic group of bovine salivary mucoprotein. Biochim Biophys Acta. 1959;34:380–391. - PubMed
-
- Weis W, Brown JH, Cusack S, Paulson JC, Skehel JJ, Wiley DC. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. - PubMed
-
- Scholtissek C, Rohde W, Von Hoyningen V, Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978;87:13–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

