A novel histidine tyrosine phosphatase, TULA-2, associates with Syk and negatively regulates GPVI signaling in platelets
- PMID: 20585042
- PMCID: PMC2953889
- DOI: 10.1182/blood-2010-02-268136
A novel histidine tyrosine phosphatase, TULA-2, associates with Syk and negatively regulates GPVI signaling in platelets
Abstract
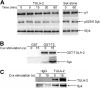
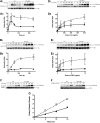
T-cell ubiquitin ligand-2 (TULA-2) is a recently discovered histidine tyrosine phosphatase thought to be ubiquitously expressed. In this work, we have investigated whether TULA-2 has a key role in platelet glycoprotein VI (GPVI) signaling. This study indicates that TULA-2 is expressed in human and murine platelets and is able to associate with Syk and dephosphorylate it. Ablation of TULA-2 resulted in hyperphosphorylation of Syk and its downstream effector phospholipase C-γ2 as well as enhanced GPVI-mediated platelet functional responses. In addition, shorter bleeding times and a prothrombotic phenotype were observed in mice lacking TULA-2. We therefore propose that TULA-2 is the primary tyrosine phosphatase mediating the dephosphorylation of Syk and thus functions as a negative regulator of GPVI signaling in platelets.
Figures
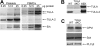




References
-
- Blockmans D, Deckmyn H, Vermylen J. Platelet activation. Blood Rev. 1995;9(3):143–156. - PubMed
-
- Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102(2):449–461. - PubMed
-
- Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J Biol Chem. 1996;271(30):18095–18099. - PubMed
-
- Fujii C, Yanagi S, Sada K, Nagai K, Taniguchi T, Yamamura H. Involvement of protein-tyrosine kinase p72syk in collagen-induced signal transduction in platelets. Eur J Biochem. 1994;226(1):243–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

