Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients
- PMID: 20585090
- PMCID: PMC2953970
- DOI: 10.1200/JCO.2009.25.9655
Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients
Abstract
Purpose: While studies have found that adjuvant hormonal therapy for hormone-sensitive breast cancer (BC) dramatically reduces recurrence and mortality, adherence to medications is suboptimal. We investigated the rates and predictors of early discontinuation and nonadherence to hormonal therapy in patients enrolled in Kaiser Permanente of Northern California health system.
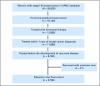
Patients and methods: We identified women diagnosed with hormone-sensitive stage I-III BC from 1996 to 2007 and used automated pharmacy records to identify hormonal therapy prescriptions and dates of refill. We used Cox proportional hazards regression models to analyze factors associated with early discontinuation and nonadherence (medication possession ratio < 80%) of hormonal therapy.
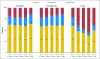
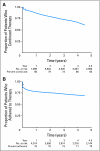
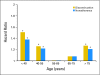
Results: We identified 8,769 patients with BC who met our eligibility criteria and who filled at least one prescription for tamoxifen (43%), aromatase inhibitors (26%), or both (30%) within 1 year of diagnosis. Younger or older age, lumpectomy (v mastectomy), and comorbidities were associated with earlier discontinuation, while Asian race, being married, earlier year at diagnosis, receipt of chemotherapy or radiotherapy, and longer prescription refill interval were associated with completion of 4.5 years of therapy. Of those who continued therapy, similar factors were associated with full adherence. Women age younger than 40 years had the highest risk of discontinuation (hazard ratio, 1.51; 95% CI, 1.23 to 1.85). By 4.5 years, 32% discontinued therapy, and of those who continued, 72% were fully adherent.
Conclusion: Only 49% of patients with BC took adjuvant hormonal therapy for the full duration at the optimal schedule. Younger women are at high risk of nonadherence. Interventions to improve adherence and continuation of hormonal therapy are needed, especially for younger women.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Compliance with tamoxifen in women with breast cancer and a BRCA1 or BRCA2 mutation.J Clin Oncol. 2010 Nov 20;28(33):e698-9; author reply e700. doi: 10.1200/JCO.2010.31.5770. Epub 2010 Oct 4. J Clin Oncol. 2010. PMID: 20921464 No abstract available.
References
-
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. - PubMed
-
- Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. - PubMed
-
- Magai C, Consedine N, Neugut AI, et al. Common psychosocial factors underlying breast cancer screening and breast cancer treatment adherence: A conceptual review and synthesis. J Womens Health (Larchmt) 2007;16:11–23. - PubMed
-
- Magai C, Consedine NS, Adjei BA, et al. Psychosocial influences on suboptimal adjuvant breast cancer treatment adherence among African American women: Implications for education and intervention. Health Educ Behav. 2008;35:835–854. - PubMed
-
- Escalada P, Griffiths P. Do people with cancer comply with oral chemotherapy treatments? Br J Community Nurs. 2006;11:532–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

