Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins
- PMID: 20585129
- PMCID: PMC2934994
- DOI: 10.1128/AAC.00380-10
Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins
Abstract
Ceragenins are cholic acid-derived antimicrobial agents that mimic the activity of endogenous antimicrobial peptides. Ceragenins target bacterial membranes, yet the consequences of these interactions have not been fully elucidated. The role of the outer membrane in allowing access of the ceragenins to the cytoplasmic membrane of gram-negative bacteria was studied using the ML-35p mutant strain of Escherichia coli that has been engineered to allow independent monitoring of small-molecule flux across the inner and outer membranes. The ceragenins CSA-8, CSA-13, and CSA-54 permeabilize the outer membrane of this bacterium, suggesting that the outer membrane does not play a major role in preventing the access of these agents to the cytoplasmic membrane. However, only the most potent of these ceragenins, CSA-13, was able to permeabilize the inner membrane. Interestingly, neither CSA-8 nor CSA-54 caused inner membrane permeabilization over a 30-min period, even at concentrations well above those required for bacterial toxicity. To further assess the role of membrane interactions, we measured membrane depolarization in gram-positive bacteria with different membrane lipid compositions, as well as in gram-negative bacteria. We found greatly increased membrane depolarization at the minimal bactericidal concentration of the ceragenins for bacterial species containing a high concentration of phosphatidylethanolamine or uncharged lipids in their cytoplasmic membranes. Although membrane lipid composition affected bactericidal efficiency, membrane depolarization was sufficient to cause lethality, providing that agents could access the cytoplasmic membrane. Consequently, we propose that in targeting bacterial cytoplasmic membranes, focus be placed on membrane depolarization as an indicator of potency.
Figures



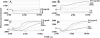
Similar articles
-
Ceragenins: cholic acid-based mimics of antimicrobial peptides.Acc Chem Res. 2008 Oct;41(10):1233-40. doi: 10.1021/ar700270t. Epub 2008 Jul 11. Acc Chem Res. 2008. PMID: 18616297
-
Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins).Biochim Biophys Acta. 2007 Oct;1768(10):2500-9. doi: 10.1016/j.bbamem.2007.05.023. Epub 2007 Jun 2. Biochim Biophys Acta. 2007. PMID: 17599802
-
Antibiofilm activities of ceragenins and antimicrobial peptides against fungal-bacterial mono and multispecies biofilms.J Antibiot (Tokyo). 2020 Jul;73(7):455-462. doi: 10.1038/s41429-020-0299-0. Epub 2020 Mar 23. J Antibiot (Tokyo). 2020. PMID: 32203127
-
Molecular mechanisms of membrane targeting antibiotics.Biochim Biophys Acta. 2016 May;1858(5):980-7. doi: 10.1016/j.bbamem.2015.10.018. Epub 2015 Oct 26. Biochim Biophys Acta. 2016. PMID: 26514603 Review.
-
Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents.Drug News Perspect. 2008 Jul-Aug;21(6):307-11. doi: 10.1358/dnp.2008.21.6.1246829. Drug News Perspect. 2008. PMID: 18836587 Review.
Cited by
-
The Role of Ovotransferrin in Egg-White Antimicrobial Activity: A Review.Foods. 2021 Apr 10;10(4):823. doi: 10.3390/foods10040823. Foods. 2021. PMID: 33920211 Free PMC article. Review.
-
The target of daptomycin is absent from Escherichia coli and other gram-negative pathogens.Antimicrob Agents Chemother. 2013 Jan;57(1):637-9. doi: 10.1128/AAC.02005-12. Epub 2012 Oct 31. Antimicrob Agents Chemother. 2013. PMID: 23114759 Free PMC article.
-
In vitro efficacy of a novel active-release antimicrobial coating to eradicate biofilms of Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2014;58(4):2400-4. doi: 10.1128/AAC.01798-13. Epub 2014 Jan 6. Antimicrob Agents Chemother. 2014. PMID: 24395238 Free PMC article.
-
Natural Antimicrobial Peptides and Their Synthetic Analogues for Effective Oral Microflora Control and Oral Infection Treatment-The Role of Ceragenins in the Development of New Therapeutic Methods.Pharmaceuticals (Basel). 2024 Dec 20;17(12):1725. doi: 10.3390/ph17121725. Pharmaceuticals (Basel). 2024. PMID: 39770567 Free PMC article. Review.
-
Membrane-active peptides and the clustering of anionic lipids.Biophys J. 2012 Jul 18;103(2):265-74. doi: 10.1016/j.bpj.2012.06.004. Epub 2012 Jul 17. Biophys J. 2012. PMID: 22853904 Free PMC article.
References
-
- Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H. G. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. - PubMed
-
- Bucki, R., A. G. Sostarecz, F. J. Byfield, P. B. Savage, and P. A. Janmey. 2007. Resistance of the antibacterial agent ceragenin CSA-13 to inactivation by DNA or F-actin and its activity in cystic fibrosis sputum. J. Antimicrob. Chemother. 60:535-545. - PubMed
-
- Chin, J. N., R. N. Jones, H. S. Sader, P. B. Savage, and M. J. Rybak. 2008. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J. Antimicrob. Chemother. 61:365-370. - PubMed
-
- Chongsiriwatana, N. P., and A. E. Barron. 2010. Comparing bacterial membrane interactions of antimicrobial peptides and their mimics. Methods Mol. Biol. 618:171-182. - PubMed
-
- Ding, B., Q. Guan, J. P. Walsh, J. S. Boswell, T. W. Winter, E. S. Winter, S. S. Boyd, C. Li, and P. B. Savage. 2002. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 45:663-669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

