Improving quinolone use in hospitals by using a bundle of interventions in an interrupted time series analysis
- PMID: 20585135
- PMCID: PMC2934965
- DOI: 10.1128/AAC.01581-09
Improving quinolone use in hospitals by using a bundle of interventions in an interrupted time series analysis
Abstract
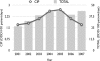
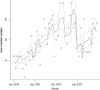
The objectives of the present study were to determine the effects of multiple targeted interventions on the level of use of quinolones and the observed rates of resistance to quinolones in Escherichia coli isolates from hospitalized patients. A bundle consisting of four interventions to improve the use of quinolones was implemented. The outcome was measured from the monthly levels of use of intravenous (i.v.) and oral quinolones and the susceptibility patterns for E. coli isolates from hospitalized patients. Statistical analyses were performed using segmented regression analysis and segmented Poisson regression models. Before the bundle was implemented, the annual use of quinolones was 2.7 defined daily doses (DDDs)/100 patient days. After the interventions, in 2007, this was reduced to 1.7 DDDs/100 patient days. The first intervention, a switch from i.v. to oral medication, was associated with a stepwise reduction in i.v. quinolone use of 71 prescribed daily doses (PDDs) per month (95% confidence interval [CI] = 47 to 95 PDDs/month, P < 0.001). Intervention 2, introduction of a new antibiotic guideline and education program, was associated with a stepwise reduction in the overall use of quinolones (reduction, 107 PDDs/month [95% CI = 58 to 156 PDDs/month). Before the interventions the quinolone resistance rate was increasing, on average, by 4.6% (95% CI = 2.6 to 6.1%) per year. This increase leveled off, which was associated with intervention 2 and intervention 4, active monitoring of prescriptions and feedback. Trends in resistance to other antimicrobial agents did not change. This study showed that the hospital-wide use of quinolones can be significantly reduced by an active policy consisting of multiple interventions. There was also a stepwise reduction in the rate of quinolone resistance associated with the bundle of interventions.
Figures


References
-
- Beaber, J. W., B. Hochhut, and M. K. Waldor. 2004. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 427:72-74. - PubMed
-
- Bruinsma, N., P. M. G. Filius, A. E. van den Bogaard, S. Nys, J. Degener, H. P. Endtz, and E. E. Stobberingh. 2003. Hospitalization, a risk factor for antibiotic-resistant Escherichia coli in the community? J. Antimicrob. Chemother. 51:1029-1032. - PubMed
-
- Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing: 15th information supplement. M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Cohn, S. M., P. A. Lipsett, T. G. Buchman, W. G. Cheaddle, J. W. Milson, S. O'Marro, A. E. Yellin, S. Jungerwirth, E. V. Rochfort, D. C. Haverstock, and S. F. Kowalsky. 2000. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann. Surg. 232:254-262. - PMC - PubMed
-
- de Marie, S., M. F. VandenBergh, S. L. Buijk, H. A. Bruining, A. van Vliet, J. A. Kluytmans, and J. W. Moutonn. 1998. Bioavailability of ciprofloxacin after multiple enteral and intravenous doses in ICU patients with severe gram-negative intra-abdominal infections. Intensive Care Med. 24:343-346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

