Clinical Use of Cinacalcet in MEN1 Hyperparathyroidism
- PMID: 20585352
- PMCID: PMC2877200
- DOI: 10.1155/2010/906163
Clinical Use of Cinacalcet in MEN1 Hyperparathyroidism
Abstract
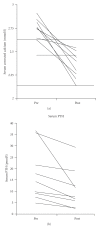
Background. Management of multiple-endocrine neoplasia type 1- (MEN1-) associated hyperparathyroidism is associated with high recurrence rates and high surgical morbidity due to multiple neck explorations. Cinacalcet, a calcimimetic agent licensed for the treatment of secondary hyperparathyroidism and parathyroid carcinoma, may provide a medical alternative for the management of these complex patients. Methods. A prospective audit was performed of eight patients; three males and five females, aged 20-38 at diagnosis. Two patients commenced cinacalcet as primary treatment and six had previous surgery. Six patients had complications of hyperparathyroidism: renal calculi, renal dysfunction, and reduced bone mineral density. All were commenced on cinacalcet 30 mg bd for MEN1 associated hyperparathyroidism; doses were subsequently reduced to 30 mg od in four patients. Results. Significant reductions were observed in serum calcium and PTH measurements. Serum calcium reduced by a median of 0.35 mmol/L (P = .012 Wilcoxon Signed Rank). Serum PTH levels decreased by a median of 5.05 pmol/L (P = .012). There was no change in urine calcium. Duration ranged from 10-35 months with maintenance of control. Cinacalcet was well tolerated by six patients; one experienced nausea and one experienced diarrhoea. Conclusion. Cinacalcet is an effective and well-tolerated medical treatment for the management of complex primary hyperparathyroidism.
Figures
Similar articles
-
Cinacalcet therapy in patients affected by primary hyperparathyroidism associated to Multiple Endocrine Neoplasia Syndrome type 1 (MEN1).Endocrine. 2016 Jun;52(3):495-506. doi: 10.1007/s12020-015-0696-5. Epub 2015 Jul 30. Endocrine. 2016. PMID: 26224587 Clinical Trial.
-
Impact of cinacalcet hydrochloride in clinical management of primary hyperparathyroidism in multiple endocrine neoplasia type 1.Minerva Endocrinol. 2013 Dec;38(4):389-94. Minerva Endocrinol. 2013. PMID: 24285106
-
A patient with MEN1-associated hyperparathyroidism, responsive to cinacalcet.Nat Clin Pract Endocrinol Metab. 2008 Jun;4(6):351-7. doi: 10.1038/ncpendmet0816. Epub 2008 Apr 15. Nat Clin Pract Endocrinol Metab. 2008. PMID: 18414463
-
Cinacalcet: An oral calcimimetic agent for the management of hyperparathyroidism.Clin Ther. 2005 Nov;27(11):1725-51. doi: 10.1016/j.clinthera.2005.11.015. Clin Ther. 2005. PMID: 16368445 Review.
-
Comparative Effectiveness of Calcimimetic Agents for Secondary Hyperparathyroidism in Adults: A Systematic Review and Network Meta-analysis.Am J Kidney Dis. 2020 Sep;76(3):321-330. doi: 10.1053/j.ajkd.2020.02.439. Epub 2020 May 28. Am J Kidney Dis. 2020. PMID: 32475604
Cited by
-
Management and Long-Term Follow-Up of Hyperparathyroidism in Multiple Endocrine Neoplasia Type 1: Single Center Experience.J Clin Med. 2022 Apr 1;11(7):1967. doi: 10.3390/jcm11071967. J Clin Med. 2022. PMID: 35407574 Free PMC article.
-
Contemporary Medical Management of Primary Hyperparathyroidism: A Systematic Review.Front Endocrinol (Lausanne). 2017 Apr 20;8:79. doi: 10.3389/fendo.2017.00079. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28473803 Free PMC article. Review.
-
Pharmacotherapy of Zollinger-Ellison syndrome.Expert Opin Pharmacother. 2013 Feb;14(3):307-21. doi: 10.1517/14656566.2013.767332. Epub 2013 Jan 30. Expert Opin Pharmacother. 2013. PMID: 23363383 Free PMC article. Review.
-
Cinacalcet therapy in patients affected by primary hyperparathyroidism associated to Multiple Endocrine Neoplasia Syndrome type 1 (MEN1).Endocrine. 2016 Jun;52(3):495-506. doi: 10.1007/s12020-015-0696-5. Epub 2015 Jul 30. Endocrine. 2016. PMID: 26224587 Clinical Trial.
-
Cinacalcet as alternative treatment for primary hyperparathyroidism: achievements and prospects.Endocrine. 2011 Jun;39(3):199-204. doi: 10.1007/s12020-011-9452-7. Epub 2011 Mar 26. Endocrine. 2011. PMID: 21442382 Review.
References
-
- Brandi ML, Gagel RF, Angeli A, et al. Consensus: guidelines for diagnosis and therapy of MEN type 1 and type 2. Journal of Clinical Endocrinology and Metabolism. 2001;86(12):5658–5671. - PubMed
-
- Burgess JR, David R, Greenaway TM, Parameswaran V, Shepherd JJ. Osteoporosis in multiple endocrine neoplasia type 1. Severity, clinical significance, relationship to primary hyperparathyroidism, and response to parathyroidectomy. Archives of Surgery. 1999;134(10):1119–1123. - PubMed
-
- Marx S. Multiple endocrine neoplasia type 1. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th edition. New York, NY, USA: McGraw-Hill; 2001.
-
- Moe SM, Cunningham J, Bommer J, et al. Long-term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HCl. Nephrology Dialysis Transplantation. 2005;20(10):2186–2193. - PubMed
-
- Silverberg SJ, Rubin MR, Faiman C, et al. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. Journal of Clinical Endocrinology and Metabolism. 2007;92(10):3803–3808. - PubMed
LinkOut - more resources
Full Text Sources

