Bronchus-associated lymphoid tissue (BALT) and survival in a vaccine mouse model of tularemia
- PMID: 20585390
- PMCID: PMC2886834
- DOI: 10.1371/journal.pone.0011156
Bronchus-associated lymphoid tissue (BALT) and survival in a vaccine mouse model of tularemia
Abstract
Background: Francisella tularensis causes severe pulmonary disease, and nasal vaccination could be the ideal measure to effectively prevent it. Nevertheless, the efficacy of this type of vaccine is influenced by the lack of an effective mucosal adjuvant.
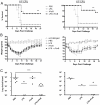
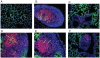
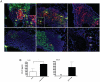
Methodology/principal findings: Mice were immunized via the nasal route with lipopolysaccharide isolated from F. tularensis and neisserial recombinant PorB as an adjuvant candidate. Then, mice were challenged via the same route with the F. tularensis attenuated live vaccine strain (LVS). Mouse survival and analysis of a number of immune parameters were conducted following intranasal challenge. Vaccination induced a systemic antibody response and 70% of mice were protected from challenge as showed by their improved survival and weight regain. Lungs from mice recovering from infection presented prominent lymphoid aggregates in peribronchial and perivascular areas, consistent with the location of bronchus-associated lymphoid tissue (BALT). BALT areas contained proliferating B and T cells, germinal centers, T cell infiltrates, dendritic cells (DCs). We also observed local production of antibody generating cells and homeostatic chemokines in BALT areas.
Conclusions: These data indicate that PorB might be an optimal adjuvant candidate for improving the protective effect of F. tularensis antigens. The presence of BALT induced after intranasal challenge in vaccinated mice might play a role in regulation of local immunity and long-term protection, but more work is needed to elucidate mechanisms that lead to its formation.
Conflict of interest statement
Figures


Similar articles
-
Neisseria meningitidis PorB, a Toll-like receptor 2 ligand, improves the capacity of Francisella tularensis lipopolysaccharide to protect mice against experimental tularemia.Clin Vaccine Immunol. 2008 Sep;15(9):1322-9. doi: 10.1128/CVI.00125-08. Epub 2008 Jul 9. Clin Vaccine Immunol. 2008. PMID: 18614668 Free PMC article.
-
Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion.Infect Immun. 2007 May;75(5):2152-62. doi: 10.1128/IAI.01606-06. Epub 2007 Feb 12. Infect Immun. 2007. PMID: 17296747 Free PMC article.
-
Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A.Infect Immun. 2005 May;73(5):2644-54. doi: 10.1128/IAI.73.5.2644-2654.2005. Infect Immun. 2005. PMID: 15845466 Free PMC article.
-
Adaptive Immunity to Francisella tularensis and Considerations for Vaccine Development.Front Cell Infect Microbiol. 2018 Apr 6;8:115. doi: 10.3389/fcimb.2018.00115. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29682484 Free PMC article. Review.
-
Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain.Microbes Infect. 2003 Feb;5(2):135-42. doi: 10.1016/s1286-4579(02)00084-9. Microbes Infect. 2003. PMID: 12650771 Review.
Cited by
-
B cell responses in older adults with latent tuberculosis: Considerations for vaccine development.Glob Vaccines Immunol. 2016 Jun;1(2):44-52. doi: 10.15761/GVI.1000112. Epub 2016 May 27. Glob Vaccines Immunol. 2016. PMID: 30271881 Free PMC article.
-
In vivo mechanisms involved in enhanced protection utilizing an Fc receptor-targeted mucosal vaccine platform in a bacterial vaccine and challenge model.Infect Immun. 2015 Jan;83(1):77-89. doi: 10.1128/IAI.02289-14. Epub 2014 Oct 13. Infect Immun. 2015. PMID: 25312957 Free PMC article.
-
Bordetella spp. block eosinophil recruitment to suppress the generation of early mucosal protection.Cell Rep. 2023 Nov 28;42(11):113294. doi: 10.1016/j.celrep.2023.113294. Epub 2023 Oct 25. Cell Rep. 2023. PMID: 37883230 Free PMC article.
-
A Unique Cellular and Molecular Microenvironment Is Present in Tertiary Lymphoid Organs of Patients with Spontaneous Prostate Cancer Regression.Front Immunol. 2017 May 17;8:563. doi: 10.3389/fimmu.2017.00563. eCollection 2017. Front Immunol. 2017. PMID: 28567040 Free PMC article.
-
The development of inducible bronchus-associated lymphoid tissue depends on IL-17.Nat Immunol. 2011 Jun 12;12(7):639-46. doi: 10.1038/ni.2053. Nat Immunol. 2011. PMID: 21666689 Free PMC article.
References
-
- Hirabayashi Y, Kurata H, Funato H, Nagamine T, Aizawa C, et al. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine. 1990;8:243–248. - PubMed
-
- Ciabattini A, Giomarelli B, Parigi R, Chiavolini D, Pettini E, et al. Intranasal immunization of mice with recombinant Streptococcus gordonii expressing NadA of Neisseria meningitidis induces systemic bactericidal antibodies and local IgA. Vaccine. 2008;26:4244–4250. - PubMed
-
- Neutra RM, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

