The involvement of IL-17A in the murine response to sub-lethal inhalational infection with Francisella tularensis
- PMID: 20585449
- PMCID: PMC2887844
- DOI: 10.1371/journal.pone.0011176
The involvement of IL-17A in the murine response to sub-lethal inhalational infection with Francisella tularensis
Abstract
Background: Francisella tularensis is an intercellular bacterium often causing fatal disease when inhaled. Previous reports have underlined the role of cell-mediated immunity and IFNgamma in the host response to Francisella tularensis infection.
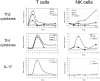
Methodology/principal findings: Here we provide evidence for the involvement of IL-17A in host defense to inhalational tularemia, using a mouse model of intranasal infection with the Live Vaccine Strain (LVS). We demonstrate the kinetics of IL-17A production in lavage fluids of infected lungs and identify the IL-17A-producing lymphocytes as pulmonary gammadelta and Th17 cells. The peak of IL-17A production appears early during sub-lethal infection, it precedes the peak of immune activation and the nadir of the disease, and then subsides subsequently. Exogenous airway administration of IL-17A or of IL-23 had a limited yet consistent effect of delaying the onset of death from a lethal dose of LVS, implying that IL-17A may be involved in restraining the infection. The protective role for IL-17A was directly demonstrated by in vivo neutralization of IL-17A. Administration of anti IL-17A antibodies concomitantly to a sub-lethal airway infection with 0.1xLD(50) resulted in a fatal disease.
Conclusion: In summary, these data characterize the involvement and underline the protective key role of the IL-17A axis in the lungs from inhalational tularemia.
Conflict of interest statement
Figures




Similar articles
-
Lung CD4-CD8- double-negative T cells are prominent producers of IL-17A and IFN-gamma during primary respiratory murine infection with Francisella tularensis live vaccine strain.J Immunol. 2010 May 15;184(10):5791-801. doi: 10.4049/jimmunol.1000362. Epub 2010 Apr 14. J Immunol. 2010. PMID: 20393138
-
Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon.Infect Immun. 2000 Apr;68(4):1988-96. doi: 10.1128/IAI.68.4.1988-1996.2000. Infect Immun. 2000. PMID: 10722593 Free PMC article.
-
Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion.Infect Immun. 2007 May;75(5):2152-62. doi: 10.1128/IAI.01606-06. Epub 2007 Feb 12. Infect Immun. 2007. PMID: 17296747 Free PMC article.
-
The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines.J Leukoc Biol. 2005 Jun;77(6):893-7. doi: 10.1189/jlb.1104637. Epub 2005 Mar 9. J Leukoc Biol. 2005. PMID: 15758077 Review.
-
Interleukin-17A: Possible mediator and therapeutic target in hypertension.Nefrologia (Engl Ed). 2021 May-Jun;41(3):244-257. doi: 10.1016/j.nefro.2020.11.009. Epub 2021 Mar 26. Nefrologia (Engl Ed). 2021. PMID: 33775443 Review. English, Spanish.
Cited by
-
Molecular immune responses to aerosol challenge with Francisella tularensis in mice inoculated with live vaccine candidates of varying efficacy.PLoS One. 2010 Oct 12;5(10):e13349. doi: 10.1371/journal.pone.0013349. PLoS One. 2010. PMID: 20967278 Free PMC article.
-
Aim2 and Nlrp3 Are Dispensable for Vaccine-Induced Immunity against Francisella tularensis Live Vaccine Strain.Infect Immun. 2021 Jun 16;89(7):e0013421. doi: 10.1128/IAI.00134-21. Epub 2021 Jun 16. Infect Immun. 2021. PMID: 33875472 Free PMC article.
-
IL-10 restrains IL-17 to limit lung pathology characteristics following pulmonary infection with Francisella tularensis live vaccine strain.Am J Pathol. 2013 Nov;183(5):1397-1404. doi: 10.1016/j.ajpath.2013.07.008. Epub 2013 Sep 3. Am J Pathol. 2013. PMID: 24007881 Free PMC article.
-
Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies.J Immunol Res. 2019 Apr 14;2019:1356540. doi: 10.1155/2019/1356540. eCollection 2019. J Immunol Res. 2019. PMID: 31111075 Free PMC article. Review.
-
Development of tolerogenic dendritic cells and regulatory T cells favors exponential bacterial growth and survival during early respiratory tularemia.J Leukoc Biol. 2011 Sep;90(3):493-507. doi: 10.1189/jlb.0411197. Epub 2011 Jul 1. J Leukoc Biol. 2011. PMID: 21724804 Free PMC article.
References
-
- Sjostedt A. Tularemia: history, epidemiology, pathogen physiology and clinical manifestations. Ann NY Acad Sci. 2007;1005:1–29. - PubMed
-
- Tarvnik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. - PubMed
-
- Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. - PubMed
-
- Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defense renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–978. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

