The Catalytic Asymmetric Intramolecular Stetter Reaction
- PMID: 20585467
- PMCID: PMC2888141
- DOI: 10.1055/s-0029-1216654
The Catalytic Asymmetric Intramolecular Stetter Reaction
Abstract
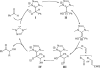
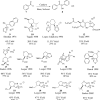
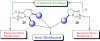
This account chronicles our efforts at the development of a catalytic asymmetric Stetter reaction using chiral triazolium salts as small molecule organic catalysts. Advances in the mechanistically related azolium-catalyzed asymmetric benzoin reaction are discussed, particularly as they apply to catalyst design. A chronological treatise of reaction discovery, catalyst optimization and reactivity extension follows.
Figures
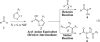



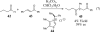
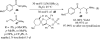
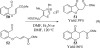
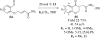
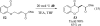


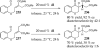


Similar articles
-
Synthesis of (1R,2R)-DPEN-derived triazolium salts and their application in asymmetric intramolecular Stetter reactions.Org Biomol Chem. 2011 Apr 7;9(7):2072-4. doi: 10.1039/c1ob00025j. Epub 2011 Feb 22. Org Biomol Chem. 2011. PMID: 21340086
-
A highly enantioselective catalytic intramolecular Stetter reaction.J Am Chem Soc. 2002 Sep 4;124(35):10298-9. doi: 10.1021/ja027411v. J Am Chem Soc. 2002. PMID: 12197730
-
Scope of the asymmetric intramolecular stetter reaction catalyzed by chiral nucleophilic triazolinylidene carbenes.J Org Chem. 2008 Mar 21;73(6):2033-40. doi: 10.1021/jo702313f. Epub 2008 Feb 27. J Org Chem. 2008. PMID: 18302407 Free PMC article.
-
Remote Electronic Tuning of Chiral N-Heterocyclic Carbenes.Chem Rec. 2023 Jul;23(7):e202300103. doi: 10.1002/tcr.202300103. Epub 2023 May 31. Chem Rec. 2023. PMID: 37255345 Review.
-
Chiral Brønsted Acid-Catalyzed Asymmetric Reaction via Vinylidene Ortho-Quinone Methides.Chemistry. 2024 Sep 2;30(49):e202402247. doi: 10.1002/chem.202402247. Epub 2024 Aug 12. Chemistry. 2024. PMID: 38923595 Review.
Cited by
-
Benzocyclobutenone synthesis exploiting acylsilanes as photofunctional directing groups.Chem Sci. 2024 Oct 25;15(46):19328-35. doi: 10.1039/d4sc05715e. Online ahead of print. Chem Sci. 2024. PMID: 39502503 Free PMC article.
-
N-heterocyclic carbene and Brønsted acid cooperative catalysis: asymmetric synthesis of trans-γ-lactams.J Am Chem Soc. 2011 Aug 17;133(32):12466-9. doi: 10.1021/ja205714g. Epub 2011 Jul 25. J Am Chem Soc. 2011. PMID: 21780842 Free PMC article.
-
Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes.Chem Rev. 2015 Sep 9;115(17):9307-87. doi: 10.1021/acs.chemrev.5b00060. Epub 2015 May 20. Chem Rev. 2015. PMID: 25992594 Free PMC article. Review.
-
N-Heterocyclic Carbene Acyl Anion Organocatalysis by Ball-Milling.ChemSusChem. 2020 Jan 9;13(1):131-135. doi: 10.1002/cssc.201902346. Epub 2019 Nov 27. ChemSusChem. 2020. PMID: 31774627 Free PMC article.
-
Asymmetric homoenolate additions to acyl phosphonates through rational design of a tailored N-heterocyclic carbene catalyst.J Am Chem Soc. 2014 Jan 8;136(1):76-9. doi: 10.1021/ja410932t. Epub 2013 Dec 17. J Am Chem Soc. 2014. PMID: 24299299 Free PMC article.
References
-
-
For reviews, see: Regitz M. Angew. Chem., Int. Ed. Engl. 1996;35:725.. Bourissou D, Guerret O, Gabbai FP, Bertrand G. Chem. Rev. 2000;100:39.. Herrmann WA. Angew. Chem. Int. Ed. 2002;41:1291..
-
-
-
For reviews, see: Enders D, Balensiefer T. Acc. Chem. Res. 2004;37:534.. Johnson JS. Angew. Chem. Int. Ed. 2004;43:1326.. Pohl M, Lingen B, Müller M. Chem. Eur. J. 2002;8:5288.. Nair V, Bindu S, Sreekumar V. Angew. Chem. Int. Ed. 2004;43:5130.. Zeitler K. Angew. Chem. Int. Ed. 2004;43:7506.. Christmann M. Angew. Chem. Int. Ed. 2005;44:2632.. Webber P, Krische MJ. Chemtracts: Org. Chem. 2007;19:262..
-
-
- Seebach D. Angew. Chem., Int. Ed. Engl. 1979;18:239.
-
-
For reviews, see: Albright JD. Tetrahedron. 1983;39:3207.. Aitken RA, Thomas AW. Adv. Heterocyclic Chem. 2001;79:89..
-
-
-
For examples of the benzoin reaction that are not referenced later in the text, see; Hachisu Y, Bode JW, Suzuki K. J. Am. Chem. Soc. 2003;125:8432.. Enders D, Niemeier O, Balensiefer T. Angew. Chem. Int. Ed. 2006;45:1463.. For examples of the benzoin reaction with acyl silanes see; Linghu X, Johnson JS. Angew. Chem. Int Ed. 2003;42:2534.. Linghu X, Potnick JR, Johnson JS. J. Am. Chem. Soc. 2004;126:3070.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
