The F-BAR protein family Actin' on the membrane
- PMID: 20585497
- PMCID: PMC2889961
- DOI: 10.4161/cib.3.2.10521
The F-BAR protein family Actin' on the membrane
Abstract
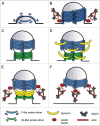
A tight spatio-temporal coordination of the machineries controlling actin dynamics and membrane remodelling is crucial for a huge variety of cellular processes that shape cells into a multicellular organism. Dynamic membrane remodelling is achieved by a functional relationship between proteins that control plasma membrane curvature, membrane fission and nucleation of new actin filaments. The BAR/F-BAR-domain-containing proteins are prime candidates to couple plasma membrane curvature and actin dynamics in different morphogenetic processes. Here, we discuss recent findings on the membrane-shaping proteins of the F-BAR domain subfamily and how they regulate morphogenetic processes in vivo.
Keywords: Cip4; F-BAR; Toca-1; WASP; WAVE; actin dynamics; cell polarity; endocytosis; membrane deformation.
Figures
Similar articles
-
Drosophila Cip4/Toca-1 integrates membrane trafficking and actin dynamics through WASP and SCAR/WAVE.Curr Biol. 2009 Sep 15;19(17):1429-37. doi: 10.1016/j.cub.2009.07.058. Epub 2009 Sep 3. Curr Biol. 2009. PMID: 19716703
-
EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization.EMBO J. 2008 Nov 5;27(21):2817-28. doi: 10.1038/emboj.2008.216. Epub 2008 Oct 16. EMBO J. 2008. PMID: 18923421 Free PMC article.
-
Members of the CIP4 family of proteins participate in the regulation of platelet-derived growth factor receptor-beta-dependent actin reorganization and migration.Biol Cell. 2010 Jan 14;102(4):215-30. doi: 10.1042/BC20090033. Biol Cell. 2010. PMID: 19909236
-
F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases.J Hematol Oncol. 2015 May 9;8:47. doi: 10.1186/s13045-015-0144-2. J Hematol Oncol. 2015. PMID: 25956236 Free PMC article. Review.
-
BAR domain proteins-a linkage between cellular membranes, signaling pathways, and the actin cytoskeleton.Biophys Rev. 2018 Dec;10(6):1587-1604. doi: 10.1007/s12551-018-0467-7. Epub 2018 Nov 19. Biophys Rev. 2018. PMID: 30456600 Free PMC article. Review.
Cited by
-
The intrinsically disordered region of the cytokinetic F-BAR protein Cdc15 performs a unique essential function in maintenance of cytokinetic ring integrity.Mol Biol Cell. 2019 Oct 15;30(22):2790-2801. doi: 10.1091/mbc.E19-06-0314. Epub 2019 Sep 11. Mol Biol Cell. 2019. PMID: 31509478 Free PMC article.
-
Adherens Junctions on the Move-Membrane Trafficking of E-Cadherin.Cold Spring Harb Perspect Biol. 2017 Mar 1;9(3):a029140. doi: 10.1101/cshperspect.a029140. Cold Spring Harb Perspect Biol. 2017. PMID: 28096264 Free PMC article. Review.
-
A complex of ZO-1 and the BAR-domain protein TOCA-1 regulates actin assembly at the tight junction.Mol Biol Cell. 2015 Aug 1;26(15):2769-87. doi: 10.1091/mbc.E15-04-0232. Epub 2015 Jun 10. Mol Biol Cell. 2015. PMID: 26063734 Free PMC article.
-
RNAi Screen in Tribolium Reveals Involvement of F-BAR Proteins in Myoblast Fusion and Visceral Muscle Morphogenesis in Insects.G3 (Bethesda). 2019 Apr 9;9(4):1141-1151. doi: 10.1534/g3.118.200996. G3 (Bethesda). 2019. PMID: 30733382 Free PMC article.
-
The cytoskeleton and neurite initiation.Bioarchitecture. 2013 Jul-Aug;3(4):86-109. doi: 10.4161/bioa.26259. Bioarchitecture. 2013. PMID: 24002528 Free PMC article. Review.
References
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. - PubMed
-
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. - PubMed
-
- Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–322. - PubMed
-
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. - PubMed
LinkOut - more resources
Full Text Sources
