F-BAR domain proteins: Families and function
- PMID: 20585502
- PMCID: PMC2889966
- DOI: 10.4161/cib.3.2.10808
F-BAR domain proteins: Families and function
Abstract
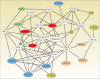
The F-BAR domain is emerging as an important player in membrane remodeling pathways. F-BAR domain proteins couple membrane remodeling with actin dynamics associated with endocytic pathways and filopodium formation. Here, we provide a comprehensive analysis of F-BAR domain proteins in terms of their evolutionary relationships and protein function. F-BAR domain containing proteins can be categorized into five subfamilies based on their phylogeny which is consistent with the additional protein domains they possess, for example, RhoGAP domains, Cdc42 binding sites, SH3 domains and tyrosine kinase domains. We derive a protein-protein interaction network suggesting that dynamin1/2, N-WASP, Huntingtin, intersectin and Cdc42 are central nodes influencing F-BAR domain protein function.
Keywords: F-BAR; endocytosis; filopodium formation; membrane trafficking; morphology.
Figures

Similar articles
-
F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases.J Hematol Oncol. 2015 May 9;8:47. doi: 10.1186/s13045-015-0144-2. J Hematol Oncol. 2015. PMID: 25956236 Free PMC article. Review.
-
I-BAR domains, IRSp53 and filopodium formation.Semin Cell Dev Biol. 2010 Jun;21(4):350-6. doi: 10.1016/j.semcdb.2009.11.008. Epub 2009 Nov 11. Semin Cell Dev Biol. 2010. PMID: 19913105 Review.
-
BAR domain proteins-a linkage between cellular membranes, signaling pathways, and the actin cytoskeleton.Biophys Rev. 2018 Dec;10(6):1587-1604. doi: 10.1007/s12551-018-0467-7. Epub 2018 Nov 19. Biophys Rev. 2018. PMID: 30456600 Free PMC article. Review.
-
Regulation of neuronal morphology by Toca-1, an F-BAR/EFC protein that induces plasma membrane invagination.J Biol Chem. 2006 Sep 29;281(39):29042-53. doi: 10.1074/jbc.M604025200. Epub 2006 Aug 2. J Biol Chem. 2006. PMID: 16885158
-
EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization.EMBO J. 2008 Nov 5;27(21):2817-28. doi: 10.1038/emboj.2008.216. Epub 2008 Oct 16. EMBO J. 2008. PMID: 18923421 Free PMC article.
Cited by
-
F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases.J Hematol Oncol. 2015 May 9;8:47. doi: 10.1186/s13045-015-0144-2. J Hematol Oncol. 2015. PMID: 25956236 Free PMC article. Review.
-
Membrane sculpting by F-BAR domains studied by molecular dynamics simulations.PLoS Comput Biol. 2013;9(1):e1002892. doi: 10.1371/journal.pcbi.1002892. Epub 2013 Jan 31. PLoS Comput Biol. 2013. PMID: 23382665 Free PMC article.
-
Impaired caveolae function and upregulation of alternative endocytic pathways induced by experimental modulation of intersectin-1s expression in mouse lung endothelium.Biochem Res Int. 2012;2012:672705. doi: 10.1155/2012/672705. Epub 2012 Feb 26. Biochem Res Int. 2012. PMID: 22506115 Free PMC article.
-
The Effects of Viral Structural Proteins on Acidic Phospholipids in Host Membranes.Viruses. 2024 Oct 31;16(11):1714. doi: 10.3390/v16111714. Viruses. 2024. PMID: 39599829 Free PMC article. Review.
-
Regulation of dynamin-2 assembly-disassembly and function through the SH3A domain of intersectin-1s.J Cell Mol Med. 2011 Nov;15(11):2364-76. doi: 10.1111/j.1582-4934.2010.01226.x. J Cell Mol Med. 2011. PMID: 21129155 Free PMC article.
References
-
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
