Somatic tetraploidy in vertebrate neurons: Implications in physiology and pathology
- PMID: 20585523
- PMCID: PMC2889987
- DOI: 10.4161/cib.3.2.11061
Somatic tetraploidy in vertebrate neurons: Implications in physiology and pathology
Abstract
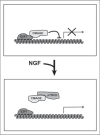
The presence of polyploid neurons in the vertebrate nervous system has been a subject of debate since the 1960s. At that time, Purkinje cells were proposed to be tetraploid, but technical limitations impeded to reach a clear conclusion, and the current belief is that most vertebrate neurons are diploid. By using up-to-date approaches we have recently demonstrated the existence of a subpopulation of tetraploid retinal ganglion cells (RGCs) in the vertebrate retina. In the chick, these neurons show large somas and extensive dendritic trees and most of them express a marker specific for RGCs innervating a specific lamina of the optic tectum. We have also demonstrated that these neurons are generated in response to nerve growth factor (NGF) acting through the neurotrophin receptor p75 (p75(NTR)), which induces E2F1 activity and cell cycle re-entry in migrating RGC neuroblasts lacking retinoblastoma (Rb) protein. We have also showed that brain-derived neurotrophic factor (BDNF) prevents G(2)/M transition in the tetraploid RGCs, thus being crucial for the maintenance of the tetraploid status as well as the survival of these neurons. The realization that tetraploid neurons can be readily observed in the vertebrate nervous system has important physiological consequences, which are discussed in this commentary.
Keywords: cell cycle; endoreduplication; nerve growth factor; p75NTR; retinal ganglion cell.
Figures
Comment on
- Morillo SM, Escoll P, de la Hera A, Frade JM. Somatic tetraploidy in specific chick retinal ganglion cells induced by nerve growth factor. Proc Natl Acad Sci USA. 2010;107:109–114.
Similar articles
-
Somatic tetraploidy in specific chick retinal ganglion cells induced by nerve growth factor.Proc Natl Acad Sci U S A. 2010 Jan 5;107(1):109-14. doi: 10.1073/pnas.0906121107. Epub 2009 Dec 14. Proc Natl Acad Sci U S A. 2010. PMID: 20018664 Free PMC article.
-
A novel hypothesis for Alzheimer disease based on neuronal tetraploidy induced by p75 (NTR).Cell Cycle. 2010 May 15;9(10):1934-41. doi: 10.4161/cc.9.10.11582. Epub 2010 May 15. Cell Cycle. 2010. PMID: 20436277 Review.
-
Effect of p75NTR on the regulation of naturally occurring cell death and retinal ganglion cell number in the mouse eye.Dev Biol. 2006 Feb 1;290(1):57-65. doi: 10.1016/j.ydbio.2005.08.051. Epub 2005 Dec 15. Dev Biol. 2006. PMID: 16343477
-
Genetic evidence for p75NTR-dependent tetraploidy in cortical projection neurons from adult mice.J Neurosci. 2013 Apr 24;33(17):7488-500. doi: 10.1523/JNEUROSCI.3849-12.2013. J Neurosci. 2013. PMID: 23616554 Free PMC article.
-
Control of neuronal ploidy during vertebrate development.Results Probl Cell Differ. 2011;53:547-63. doi: 10.1007/978-3-642-19065-0_22. Results Probl Cell Differ. 2011. PMID: 21630159 Review.
Cited by
-
Mechanisms and consequences of aneuploidy and chromosome instability in the aging brain.Mech Ageing Dev. 2017 Jan;161(Pt A):19-36. doi: 10.1016/j.mad.2016.03.007. Epub 2016 Mar 21. Mech Ageing Dev. 2017. PMID: 27013377 Free PMC article. Review.
-
Mice With Increased Numbers of Polyploid Hepatocytes Maintain Regenerative Capacity But Develop Fewer Hepatocellular Carcinomas Following Chronic Liver Injury.Gastroenterology. 2020 May;158(6):1698-1712.e14. doi: 10.1053/j.gastro.2020.01.026. Epub 2020 Jan 20. Gastroenterology. 2020. PMID: 31972235 Free PMC article.
-
A direct comparison of interphase FISH versus low-coverage single cell sequencing to detect aneuploidy reveals respective strengths and weaknesses.Sci Rep. 2019 Jul 19;9(1):10508. doi: 10.1038/s41598-019-46606-w. Sci Rep. 2019. PMID: 31324840 Free PMC article.
-
The origins and functions of hepatic polyploidy.Cell Cycle. 2019 Jun;18(12):1302-1315. doi: 10.1080/15384101.2019.1618123. Epub 2019 May 26. Cell Cycle. 2019. PMID: 31096847 Free PMC article. Review.
-
Neuronal injury external to the retina rapidly activates retinal glia, followed by elevation of markers for cell cycle re-entry and death in retinal ganglion cells.PLoS One. 2014 Jul 1;9(7):e101349. doi: 10.1371/journal.pone.0101349. eCollection 2014. PLoS One. 2014. PMID: 24983470 Free PMC article.
References
-
- Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: more for less. Cell. 2001;105:297–306. - PubMed
-
- Coggeshall RE, Yaksta BA, Swartz FJ. A cytophotometric analysis of the DNA in the nucleus of the giant cell, R-2, in Aplysia. Chromosoma. 1970;32:205–212. - PubMed
-
- Manfredi Romanini MG, Fraschini A, Bernocchi G. DNA content and nuclear area in the neurons of the cerebral ganglion in Helix pomatia. Ann Histochim. 1973;18:49–58. - PubMed
-
- Swift H. Quantitative aspects of nuclear nucleoproteins. Int Rev Cytol. 1953;2:1–76.
LinkOut - more resources
Full Text Sources
Research Materials
