NeuroML: a language for describing data driven models of neurons and networks with a high degree of biological detail
- PMID: 20585541
- PMCID: PMC2887454
- DOI: 10.1371/journal.pcbi.1000815
NeuroML: a language for describing data driven models of neurons and networks with a high degree of biological detail
Abstract
Biologically detailed single neuron and network models are important for understanding how ion channels, synapses and anatomical connectivity underlie the complex electrical behavior of the brain. While neuronal simulators such as NEURON, GENESIS, MOOSE, NEST, and PSICS facilitate the development of these data-driven neuronal models, the specialized languages they employ are generally not interoperable, limiting model accessibility and preventing reuse of model components and cross-simulator validation. To overcome these problems we have used an Open Source software approach to develop NeuroML, a neuronal model description language based on XML (Extensible Markup Language). This enables these detailed models and their components to be defined in a standalone form, allowing them to be used across multiple simulators and archived in a standardized format. Here we describe the structure of NeuroML and demonstrate its scope by converting into NeuroML models of a number of different voltage- and ligand-gated conductances, models of electrical coupling, synaptic transmission and short-term plasticity, together with morphologically detailed models of individual neurons. We have also used these NeuroML-based components to develop an highly detailed cortical network model. NeuroML-based model descriptions were validated by demonstrating similar model behavior across five independently developed simulators. Although our results confirm that simulations run on different simulators converge, they reveal limits to model interoperability, by showing that for some models convergence only occurs at high levels of spatial and temporal discretisation, when the computational overhead is high. Our development of NeuroML as a common description language for biophysically detailed neuronal and network models enables interoperability across multiple simulation environments, thereby improving model transparency, accessibility and reuse in computational neuroscience.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

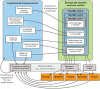

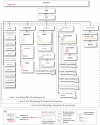
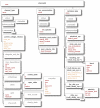
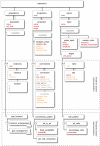

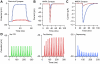


References
-
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. - PubMed
-
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, et al. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. - PubMed
-
- Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci. 2005;8:1667–1676. - PubMed
Publication types
MeSH terms
Grants and funding
- 086699/WT_/Wellcome Trust/United Kingdom
- R01 NS011613/NS/NINDS NIH HHS/United States
- R01 MH081905/MH/NIMH NIH HHS/United States
- P01 DC04732/DC/NIDCD NIH HHS/United States
- R01 DC009977/DC/NIDCD NIH HHS/United States
- R01 MH086638/MH/NIMH NIH HHS/United States
- R01 NS11613/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- BB/F005490/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0400598/MRC_/Medical Research Council/United Kingdom
- P01 DC004732/DC/NIDCD NIH HHS/United States
- 0064413/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

