The cyclin-A CYCA1;2/TAM is required for the meiosis I to meiosis II transition and cooperates with OSD1 for the prophase to first meiotic division transition
- PMID: 20585549
- PMCID: PMC2887465
- DOI: 10.1371/journal.pgen.1000989
The cyclin-A CYCA1;2/TAM is required for the meiosis I to meiosis II transition and cooperates with OSD1 for the prophase to first meiotic division transition
Abstract
Meiosis halves the chromosome number because its two divisions follow a single round of DNA replication. This process involves two cell transitions, the transition from prophase to the first meiotic division (meiosis I) and the unique meiosis I to meiosis II transition. We show here that the A-type cyclin CYCA1;2/TAM plays a major role in both transitions in Arabidopsis. A series of tam mutants failed to enter meiosis II and thus produced diploid spores and functional diploid gametes. These diploid gametes had a recombined genotype produced through the single meiosis I division. In addition, by combining the tam-2 mutation with AtSpo11-1 and Atrec8, we obtained plants producing diploid gametes through a mitotic-like division that were genetically identical to their parents. Thus tam alleles displayed phenotypes very similar to that of the previously described osd1 mutant. Combining tam and osd1 mutations leads to a failure in the prophase to meiosis I transition during male meiosis and to the production of tetraploid spores and gametes. This suggests that TAM and OSD1 are involved in the control of both meiotic transitions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




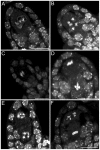

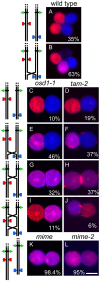





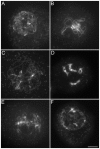
Similar articles
-
OSD1 promotes meiotic progression via APC/C inhibition and forms a regulatory network with TDM and CYCA1;2/TAM.PLoS Genet. 2012;8(7):e1002865. doi: 10.1371/journal.pgen.1002865. Epub 2012 Jul 26. PLoS Genet. 2012. PMID: 22844260 Free PMC article.
-
Meiotic progression in Arabidopsis is governed by complex regulatory interactions between SMG7, TDM1, and the meiosis I-specific cyclin TAM.Plant Cell. 2010 Nov;22(11):3791-803. doi: 10.1105/tpc.110.078378. Epub 2010 Nov 30. Plant Cell. 2010. PMID: 21119056 Free PMC article.
-
Progression through meiosis I and meiosis II in Arabidopsis anthers is regulated by an A-type cyclin predominately expressed in prophase I.Plant Physiol. 2004 Dec;136(4):4127-35. doi: 10.1104/pp.104.051201. Epub 2004 Nov 19. Plant Physiol. 2004. PMID: 15557098 Free PMC article.
-
Centromere pairing precedes meiotic chromosome pairing in plants.Sci China Life Sci. 2017 Nov;60(11):1197-1202. doi: 10.1007/s11427-017-9109-y. Epub 2017 Jul 26. Sci China Life Sci. 2017. PMID: 28755295 Review.
-
Meiotic prophase-like pathway for cleavage-independent removal of cohesin for chromosome morphogenesis.Curr Genet. 2019 Aug;65(4):817-827. doi: 10.1007/s00294-019-00959-x. Epub 2019 Mar 28. Curr Genet. 2019. PMID: 30923890 Review.
Cited by
-
Advances Towards How Meiotic Recombination Is Initiated: A Comparative View and Perspectives for Plant Meiosis Research.Int J Mol Sci. 2019 Sep 23;20(19):4718. doi: 10.3390/ijms20194718. Int J Mol Sci. 2019. PMID: 31547623 Free PMC article. Review.
-
Transcriptome Analysis of Young Ovaries Reveals Candidate Genes Involved in Gamete Formation in Lantana camara.Plants (Basel). 2019 Aug 2;8(8):263. doi: 10.3390/plants8080263. Plants (Basel). 2019. PMID: 31382394 Free PMC article.
-
A xylem-produced peptide PtrCLE20 inhibits vascular cambium activity in Populus.Plant Biotechnol J. 2020 Jan;18(1):195-206. doi: 10.1111/pbi.13187. Epub 2019 Jul 9. Plant Biotechnol J. 2020. PMID: 31199056 Free PMC article.
-
The RTR complex as caretaker of genome stability and its unique meiotic function in plants.Front Plant Sci. 2014 Feb 12;5:33. doi: 10.3389/fpls.2014.00033. eCollection 2014. Front Plant Sci. 2014. PMID: 24575106 Free PMC article. Review.
-
Apomixis in plant reproduction: a novel perspective on an old dilemma.Plant Reprod. 2013 Sep;26(3):159-79. doi: 10.1007/s00497-013-0222-y. Epub 2013 Jul 14. Plant Reprod. 2013. PMID: 23852378 Free PMC article. Review.
References
-
- Gerton JL, Hawley RS. Homologous chromosome interactions in meiosis: diversity amidst conservation. Nat Rev Genet. 2005;6:477–487. - PubMed
-
- Zickler D, Kleckner N. Meiotic chromosomes: integrating structure and function. Annu Rev Genet. 1999;33:603–754. - PubMed
-
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370:68–71. - PubMed
-
- Bretagnolle F, Thompson J. Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytologist. 1995;129:1–22. - PubMed
-
- Otto SP. The evolutionary consequences of polyploidy. Cell. 2007;131:452–462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

