Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator
- PMID: 20585552
- PMCID: PMC2887468
- DOI: 10.1371/journal.ppat.1000899
Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator
Abstract
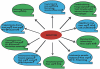
Arginine is a crucial amino acid that serves to modulate the cellular immune response during infection. Arginine is also a common substrate for both inducible nitric oxide synthase (iNOS) and arginase. The generation of nitric oxide from arginine is responsible for efficient immune response and cytotoxicity of host cells to kill the invading pathogens. On the other hand, the conversion of arginine to ornithine and urea via the arginase pathway can support the growth of bacterial and parasitic pathogens. The competition between iNOS and arginase for arginine can thus contribute to the outcome of several parasitic and bacterial infections. There are two isoforms of vertebrate arginase, both of which catalyze the conversion of arginine to ornithine and urea, but they differ with regard to tissue distribution and subcellular localization. In the case of infection with Mycobacterium, Leishmania, Trypanosoma, Helicobacter, Schistosoma, and Salmonella spp., arginase isoforms have been shown to modulate the pathology of infection by various means. Despite the existence of a considerable body of evidence about mammalian arginine metabolism and its role in immunology, the critical choice to divert the host arginine pool by pathogenic organisms as a survival strategy is still a mystery in infection biology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
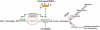
References
-
- Kossel A, Dakin HD. Über die Arginase. Z Physiol Chem. 1904;41:321–331.
-
- Mori M. Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J Nutr. 2007;137:1616S–1620S. - PubMed
-
- Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo P, et al. Arginases I and II: do their functions overlap? Mol Genet Metab. 2004;81(Suppl 1):S38–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

