A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics
- PMID: 20585561
- PMCID: PMC2887478
- DOI: 10.1371/journal.ppat.1000954
A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics
Abstract
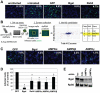
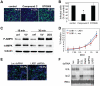
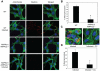
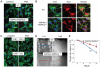
Poxviruses include medically important human pathogens, yet little is known about the specific cellular factors essential for their replication. To identify genes essential for poxvirus infection, we used high-throughput RNA interference to screen the Drosophila kinome for factors required for vaccinia infection. We identified seven genes including the three subunits of AMPK as promoting vaccinia infection. AMPK not only facilitated infection in insect cells, but also in mammalian cells. Moreover, we found that AMPK is required for macropinocytosis, a major endocytic entry pathway for vaccinia. Furthermore, we show that AMPK contributes to other virus-independent actin-dependent processes including lamellipodia formation and wound healing, independent of the known AMPK activators LKB1 and CaMKK. Therefore, AMPK plays a highly conserved role in poxvirus infection and actin dynamics independent of its role as an energy regulator.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
The Vaccinia Virus (VACV) B1 and Cellular VRK2 Kinases Promote VACV Replication Factory Formation through Phosphorylation-Dependent Inhibition of VACV B12.J Virol. 2019 Sep 30;93(20):e00855-19. doi: 10.1128/JVI.00855-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31341052 Free PMC article.
-
Labeling and Single-Particle-Tracking-Based Entry Mechanism Study of Vaccinia Virus from the Tiantan Strain.Anal Chem. 2018 Mar 6;90(5):3452-3459. doi: 10.1021/acs.analchem.7b05183. Epub 2018 Feb 14. Anal Chem. 2018. PMID: 29392930
-
Casein kinase 2 regulates vaccinia virus actin tail formation.Virology. 2012 Feb 20;423(2):143-51. doi: 10.1016/j.virol.2011.12.003. Epub 2011 Dec 29. Virology. 2012. PMID: 22209233
-
LKB1 and AMPK in cell polarity and division.Trends Cell Biol. 2008 Apr;18(4):193-8. doi: 10.1016/j.tcb.2008.01.008. Epub 2008 Mar 7. Trends Cell Biol. 2008. PMID: 18314332 Review.
-
A role for the small GTPase Rac1 in vaccinia actin-based motility.Small GTPases. 2015;6(2):119-22. doi: 10.1080/21541248.2015.1055182. Small GTPases. 2015. PMID: 26147090 Free PMC article. Review.
Cited by
-
Virus-induced translational arrest through 4EBP1/2-dependent decay of 5'-TOP mRNAs restricts viral infection.Proc Natl Acad Sci U S A. 2015 Jun 2;112(22):E2920-9. doi: 10.1073/pnas.1418805112. Epub 2015 May 18. Proc Natl Acad Sci U S A. 2015. PMID: 26038567 Free PMC article.
-
Connecting metabolism to intestinal barrier function: The role of leptin.Tissue Barriers. 2014 Aug 8;2(4):e970940. doi: 10.4161/21688362.2014.970940. eCollection 2014. Tissue Barriers. 2014. PMID: 25610758 Free PMC article.
-
Cell entry of enveloped viruses.Adv Genet. 2011;73:121-83. doi: 10.1016/B978-0-12-380860-8.00004-5. Adv Genet. 2011. PMID: 21310296 Free PMC article. Review.
-
Triad of human cellular proteins, IRF2, FAM111A, and RFC3, restrict replication of orthopoxvirus SPI-1 host-range mutants.Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):3720-3725. doi: 10.1073/pnas.1700678114. Epub 2017 Mar 20. Proc Natl Acad Sci U S A. 2017. PMID: 28320935 Free PMC article.
-
Viruses and antiviral immunity in Drosophila.Dev Comp Immunol. 2014 Jan;42(1):67-84. doi: 10.1016/j.dci.2013.05.002. Epub 2013 May 13. Dev Comp Immunol. 2014. PMID: 23680639 Free PMC article. Review.
References
-
- Moss B. Poxviridae: The viruses and their replication. In: Howley DMKaPM., editor. Fields Virology. Philadelphia: Lippincott, Williams, and Wilkins; 2001. pp. 2849–2884.
-
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

