Tetherin restricts productive HIV-1 cell-to-cell transmission
- PMID: 20585562
- PMCID: PMC2887479
- DOI: 10.1371/journal.ppat.1000955
Tetherin restricts productive HIV-1 cell-to-cell transmission
Abstract
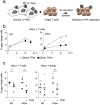
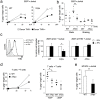
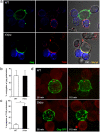
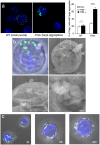
The IFN-inducible antiviral protein tetherin (or BST-2/CD317/HM1.24) impairs release of mature HIV-1 particles from infected cells. HIV-1 Vpu antagonizes the effect of tetherin. The fate of virions trapped at the cell surface remains poorly understood. Here, we asked whether tetherin impairs HIV cell-to-cell transmission, a major means of viral spread. Tetherin-positive or -negative cells, infected with wild-type or DeltaVpu HIV, were used as donor cells and cocultivated with target lymphocytes. We show that tetherin inhibits productive cell-to-cell transmission of DeltaVpu to targets and impairs that of WT HIV. Tetherin accumulates with Gag at the contact zone between infected and target cells, but does not prevent the formation of virological synapses. In the presence of tetherin, viruses are then mostly transferred to targets as abnormally large patches. These viral aggregates do not efficiently promote infection after transfer, because they accumulate at the surface of target cells and are impaired in their fusion capacities. Tetherin, by imprinting virions in donor cells, is the first example of a surface restriction factor limiting viral cell-to-cell spread.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Tetherin/BST-2: Restriction Factor or Immunomodulator?Curr HIV Res. 2016;14(3):235-46. doi: 10.2174/1570162x14999160224102752. Curr HIV Res. 2016. PMID: 26957198 Free PMC article. Review.
-
Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells.J Virol. 2010 Dec;84(23):12185-99. doi: 10.1128/JVI.01447-10. Epub 2010 Sep 22. J Virol. 2010. PMID: 20861257 Free PMC article.
-
Tetherin restricts direct cell-to-cell infection of HIV-1.Retrovirology. 2010 Dec 24;7:115. doi: 10.1186/1742-4690-7-115. Retrovirology. 2010. PMID: 21184674 Free PMC article.
-
HIV-1 Vpu Antagonizes CD317/Tetherin by Adaptor Protein-1-Mediated Exclusion from Virus Assembly Sites.J Virol. 2016 Jul 11;90(15):6709-6723. doi: 10.1128/JVI.00504-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27170757 Free PMC article.
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
Cited by
-
The HIV-1 Vpu viroporin inhibitor BIT225 does not affect Vpu-mediated tetherin antagonism.PLoS One. 2011;6(11):e27660. doi: 10.1371/journal.pone.0027660. Epub 2011 Nov 14. PLoS One. 2011. PMID: 22110710 Free PMC article.
-
HIV-infected T cells are migratory vehicles for viral dissemination.Nature. 2012 Oct 11;490(7419):283-7. doi: 10.1038/nature11398. Epub 2012 Aug 1. Nature. 2012. PMID: 22854780 Free PMC article.
-
Antibody Neutralization of HIV-1 Crossing the Blood-Brain Barrier.mBio. 2020 Oct 20;11(5):e02424-20. doi: 10.1128/mBio.02424-20. mBio. 2020. PMID: 33082263 Free PMC article.
-
A single nucleotide polymorphism in tetherin promotes retrovirus restriction in vivo.PLoS Pathog. 2012;8(3):e1002596. doi: 10.1371/journal.ppat.1002596. Epub 2012 Mar 22. PLoS Pathog. 2012. PMID: 22457621 Free PMC article.
-
Tetherin/BST-2: Restriction Factor or Immunomodulator?Curr HIV Res. 2016;14(3):235-46. doi: 10.2174/1570162x14999160224102752. Curr HIV Res. 2016. PMID: 26957198 Free PMC article. Review.
References
-
- Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. - PubMed
-
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

