Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy
- PMID: 20585569
- PMCID: PMC2891838
- DOI: 10.1371/journal.ppat.1000963
Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy
Abstract
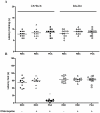
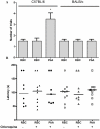
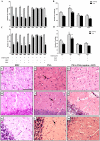
Neurological impairments are frequently detected in children surviving cerebral malaria (CM), the most severe neurological complication of infection with Plasmodium falciparum. The pathophysiology and therapy of long lasting cognitive deficits in malaria patients after treatment of the parasitic disease is a critical area of investigation. In the present study we used several models of experimental malaria with differential features to investigate persistent cognitive damage after rescue treatment. Infection of C57BL/6 and Swiss (SW) mice with Plasmodium berghei ANKA (PbA) or a lethal strain of Plasmodium yoelii XL (PyXL), respectively, resulted in documented CM and sustained persistent cognitive damage detected by a battery of behavioral tests after cure of the acute parasitic disease with chloroquine therapy. Strikingly, cognitive impairment was still present 30 days after the initial infection. In contrast, BALB/c mice infected with PbA, C57BL6 infected with Plasmodium chabaudi chabaudi and SW infected with non lethal Plasmodium yoelii NXL (PyNXL) did not develop signs of CM, were cured of the acute parasitic infection by chloroquine, and showed no persistent cognitive impairment. Reactive oxygen species have been reported to mediate neurological injury in CM. Increased production of malondialdehyde (MDA) and conjugated dienes was detected in the brains of PbA-infected C57BL/6 mice with CM, indicating high oxidative stress. Treatment of PbA-infected C57BL/6 mice with additive antioxidants together with chloroquine at the first signs of CM prevented the development of persistent cognitive damage. These studies provide new insights into the natural history of cognitive dysfunction after rescue therapy for CM that may have clinical relevance, and may also be relevant to cerebral sequelae of sepsis and other disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

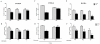
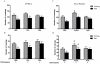
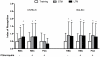
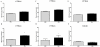
Similar articles
-
Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria.PLoS Pathog. 2012 Dec;8(12):e1003099. doi: 10.1371/journal.ppat.1003099. Epub 2012 Dec 27. PLoS Pathog. 2012. PMID: 23300448 Free PMC article.
-
Differential role of T regulatory and Th17 in Swiss mice infected with Plasmodium berghei ANKA and Plasmodium yoelii.Exp Parasitol. 2014 Jun;141:82-92. doi: 10.1016/j.exppara.2014.03.003. Epub 2014 Mar 24. Exp Parasitol. 2014. PMID: 24675415
-
A Neuroprotective Effect of the Glutamate Receptor Antagonist MK801 on Long-Term Cognitive and Behavioral Outcomes Secondary to Experimental Cerebral Malaria.Mol Neurobiol. 2017 Nov;54(9):7063-7082. doi: 10.1007/s12035-016-0226-3. Epub 2016 Oct 28. Mol Neurobiol. 2017. PMID: 27796746
-
Vascular dysfunction as a target for adjuvant therapy in cerebral malaria.Mem Inst Oswaldo Cruz. 2014 Aug;109(5):577-88. doi: 10.1590/0074-0276140061. Mem Inst Oswaldo Cruz. 2014. PMID: 25185000 Free PMC article. Review.
-
Unveiling new perspectives about the onset of neurological and cognitive deficits in cerebral malaria: exploring cellular and neurochemical mechanisms.Front Cell Infect Microbiol. 2025 Feb 6;15:1506282. doi: 10.3389/fcimb.2025.1506282. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 39981376 Free PMC article. Review.
Cited by
-
Integrin αDβ2 (CD11d/CD18) mediates experimental malaria-associated acute respiratory distress syndrome (MA-ARDS).Malar J. 2016 Jul 30;15(1):393. doi: 10.1186/s12936-016-1447-7. Malar J. 2016. PMID: 27473068 Free PMC article.
-
Non-cerebral malaria: does such a thing exist?Mem Inst Oswaldo Cruz. 2025 Feb 3;120:e240223. doi: 10.1590/0074-02760240223. eCollection 2025. Mem Inst Oswaldo Cruz. 2025. PMID: 39907418 Free PMC article. Review.
-
N-acetyl cysteine and mushroom Agaricus sylvaticus supplementation decreased parasitaemia and pulmonary oxidative stress in a mice model of malaria.Malar J. 2015 May 15;14:202. doi: 10.1186/s12936-015-0717-0. Malar J. 2015. PMID: 25971771 Free PMC article.
-
Modulation of cerebral malaria by curcumin as an adjunctive therapy.Braz J Infect Dis. 2013 Sep-Oct;17(5):579-91. doi: 10.1016/j.bjid.2013.03.004. Epub 2013 Jul 29. Braz J Infect Dis. 2013. PMID: 23906771 Free PMC article. Review.
-
The novel ETA receptor antagonist HJP-272 prevents cerebral microvascular hemorrhage in cerebral malaria and synergistically improves survival in combination with an artemisinin derivative.Life Sci. 2012 Oct 15;91(13-14):687-92. doi: 10.1016/j.lfs.2012.07.006. Epub 2012 Jul 17. Life Sci. 2012. PMID: 22820174 Free PMC article.
References
-
- Hunt NH, Golenser J, Chan-Ling T, Parekh S, Rae C, et al. Immunopathogenesis of cerebral malaria. Int J Parasitol. 2006;36:569–582. - PubMed
-
- Golenser J, McQuillan J, Hee L, Mitchell AJ, Hunt NH. Conventional and experimental treatment of cerebral malaria. Int J Parasitol. 2006;36:583–593. - PubMed
-
- van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. J Pediatr. 1997;131:125–129. - PubMed
-
- Carter JA, Lees JA, Gona JK, Murira G, Rimba K, et al. Severe falciparum malaria and acquired childhood language disorder. Dev Med Child Neurol. 2006;48:51–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

