Role of PKR and Type I IFNs in viral control during primary and secondary infection
- PMID: 20585572
- PMCID: PMC2891951
- DOI: 10.1371/journal.ppat.1000966
Role of PKR and Type I IFNs in viral control during primary and secondary infection
Abstract
Type I interferons (IFNs) are known to mediate viral control, and also promote survival and expansion of virus-specific CD8+ T cells. However, it is unclear whether signaling cascades involved in eliciting these diverse cellular effects are also distinct. One of the best-characterized anti-viral signaling mechanisms of Type I IFNs is mediated by the IFN-inducible dsRNA activated protein kinase, PKR. Here, we have investigated the role of PKR and Type I IFNs in regulating viral clearance and CD8+ T cell response during primary and secondary viral infections. Our studies demonstrate differential requirement for PKR, in viral control versus elicitation of CD8+ T cell responses during primary infection of mice with lymphocytic choriomeningitis virus (LCMV). PKR-deficient mice mounted potent CD8+ T cell responses, but failed to effectively control LCMV. The compromised LCMV control in the absence of PKR was multifactorial, and linked to less effective CD8+ T cell-mediated viral suppression, enhanced viral replication in cells, and lower steady state expression levels of IFN-responsive genes. Moreover, we show that despite normal expansion of memory CD8+ T cells and differentiation into effectors during a secondary response, effective clearance of LCMV but not vaccinia virus required PKR activity in infected cells. In the absence of Type I IFN signaling, secondary effector CD8+ T cells were ineffective in controlling both LCMV and vaccinia virus replication in vivo. These findings provide insight into cellular pathways of Type I IFN actions, and highlight the under-appreciated importance of innate immune mechanisms of viral control during secondary infections, despite the accelerated responses of memory CD8+ T cells. Additionally, the results presented here have furthered our understanding of the immune correlates of anti-viral protective immunity, which have implications in the rational design of vaccines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

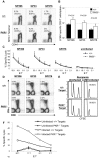
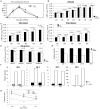




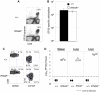

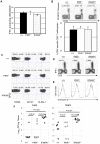
Comment in
-
Effective control of viral infections by the adaptive immune system requires assistance from innate immunity.Expert Rev Vaccines. 2010 Oct;9(10):1143-7. doi: 10.1586/erv.10.119. Expert Rev Vaccines. 2010. PMID: 20923266
Similar articles
-
Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation.J Immunol. 2006 Aug 1;177(3):1746-54. doi: 10.4049/jimmunol.177.3.1746. J Immunol. 2006. PMID: 16849484
-
Role of interferon regulatory factor 7 in T cell responses during acute lymphocytic choriomeningitis virus infection.J Virol. 2012 Oct;86(20):11254-65. doi: 10.1128/JVI.00576-12. Epub 2012 Aug 8. J Virol. 2012. PMID: 22875973 Free PMC article.
-
Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection.J Immunol. 2013 Mar 1;190(5):1991-2000. doi: 10.4049/jimmunol.1202379. Epub 2013 Jan 25. J Immunol. 2013. PMID: 23355736 Free PMC article.
-
Confounding roles for type I interferons during bacterial and viral pathogenesis.Int Immunol. 2013 Dec;25(12):663-9. doi: 10.1093/intimm/dxt050. Epub 2013 Oct 24. Int Immunol. 2013. PMID: 24158954 Free PMC article. Review.
-
Lymphocytic choriomeningitis infection of the central nervous system.Front Biosci. 2008 May 1;13:4529-43. doi: 10.2741/3021. Front Biosci. 2008. PMID: 18508527 Free PMC article. Review.
Cited by
-
The protein kinase double-stranded RNA-dependent (PKR) enhances protection against disease cause by a non-viral pathogen.PLoS Pathog. 2013;9(8):e1003557. doi: 10.1371/journal.ppat.1003557. Epub 2013 Aug 22. PLoS Pathog. 2013. PMID: 23990781 Free PMC article.
-
Post-transcriptional coordination of immunological responses by RNA-binding proteins.Nat Immunol. 2014 Jun;15(6):492-502. doi: 10.1038/ni.2884. Nat Immunol. 2014. PMID: 24840980 Review.
-
Protein Kinase R in Bacterial Infections: Friend or Foe?Front Immunol. 2021 Jul 8;12:702142. doi: 10.3389/fimmu.2021.702142. eCollection 2021. Front Immunol. 2021. PMID: 34305942 Free PMC article. Review.
-
The Antiviral Compound PSP Inhibits HIV-1 Entry via PKR-Dependent Activation in Monocytic Cells.Viruses. 2023 Mar 22;15(3):804. doi: 10.3390/v15030804. Viruses. 2023. PMID: 36992512 Free PMC article.
-
Post-transcriptional Regulation of Immunological Responses through Riboclustering.Front Immunol. 2016 Apr 29;7:161. doi: 10.3389/fimmu.2016.00161. eCollection 2016. Front Immunol. 2016. PMID: 27199986 Free PMC article. Review.
References
-
- Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. - PubMed
-
- Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8:113–126. - PubMed
-
- Biron CA. Interferons alpha and beta as immune regulators–a new look. Immunity. 2001;14:661–664. - PubMed
-
- Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176:3315–3319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

