A femtomol range FRET biosensor reports exceedingly low levels of cell surface furin: implications for the processing of anthrax protective antigen
- PMID: 20585585
- PMCID: PMC2892035
- DOI: 10.1371/journal.pone.0011305
A femtomol range FRET biosensor reports exceedingly low levels of cell surface furin: implications for the processing of anthrax protective antigen
Retraction in
-
Retraction: A Femtomol Range FRET Biosensor Reports Exceedingly Low Levels of Cell Surface Furin: Implications for the Processing of Anthrax Protective Antigen.PLoS One. 2022 May 26;17(5):e0269294. doi: 10.1371/journal.pone.0269294. eCollection 2022. PLoS One. 2022. PMID: 35617259 Free PMC article. No abstract available.
Abstract
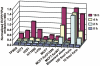
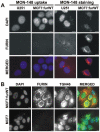
Furin, a specialized endoproteinase, transforms proproteins into biologically active proteins. Furin function is important for normal cells and also in multiple pathologies including malignancy and anthrax. Furin is believed to cycle between the Golgi compartment and the cell surface. Processing of anthrax protective antigen-83 (PA83) by the cells is considered thus far as evidence for the presence of substantial levels of cell-surface furin. To monitor furin, we designed a cleavage-activated FRET biosensor in which the Enhanced Cyan and Yellow Fluorescent Proteins were linked by the peptide sequence SNSRKKR / STSAGP derived from anthrax PA83. Both because of the sensitivity and selectivity of the anthrax sequence to furin proteolysis and the FRET-based detection, the biosensor recorded the femtomolar levels of furin in the in vitro reactions and cell-based assays. Using the biosensor that was cell-impermeable because of its size and also by other relevant methods, we determined that exceedingly low levels, if any, of cell-surface furin are present in the intact cells and in the cells with the enforced furin overexpression. This observation was in a sharp contrast with the existing concepts about the furin presentation on cell surfaces and anthrax disease mechanism. We next demonstrated using cell-based tests that PA83, in fact, was processed by furin in the extracellular milieu and that only then the resulting PA63 bound the anthrax toxin cell-surface receptors. We also determined that the biosensor, but not the conventional peptide substrates, allowed continuous monitoring of furin activity in cancer cell extracts. Our results suggest that there are no physiologically-relevant levels of cell-surface furin and, accordingly, that the mechanisms of anthrax should be re-investigated. In addition, the availability of the biosensor is a foundation for non-invasive monitoring of furin activity in cancer cells. Conceptually, the biosensor we developed may serve as a prototype for other proteinase-activated biosensors.
Conflict of interest statement
Figures
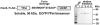
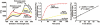
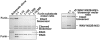
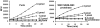
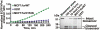
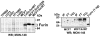
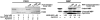

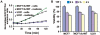
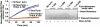
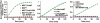
References
-
- Ai HW, Hazelwood KL, Davidson MW, Campbell RE. Fluorescent protein FRET pairs for ratiometric imaging of dual biosensors. Nat Methods. 2008;5:401–403. - PubMed
-
- Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2:905–909. - PubMed
-
- Wang Y, Shyy JY, Chien S. Fluorescence proteins, live-cell imaging, and mechanobiology: seeing is believing. Annu Rev Biomed Eng. 2008;10:1–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

