The desmosomal plaque proteins of the plakophilin family
- PMID: 20585595
- PMCID: PMC2879962
- DOI: 10.1155/2010/101452
The desmosomal plaque proteins of the plakophilin family
Abstract
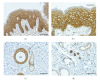
Three related proteins of the plakophilin family (PKP1_3) have been identified as junctional proteins that are essential for the formation and stabilization of desmosomal cell contacts. Failure of PKP expression can have fatal effects on desmosomal adhesion, leading to abnormal tissue and organ development. Thus, loss of functional PKP 1 in humans leads to ectodermal dysplasia/skin fragility (EDSF) syndrome, a genodermatosis with severe blistering of the epidermis as well as abnormal keratinocytes differentiation. Mutations in the human PKP 2 gene have been linked to severe heart abnormalities that lead to arrhythmogenic right ventricular cardiomyopathy (ARVC). In the past few years it has been shown that junctional adhesion is not the only function of PKPs. These proteins have been implicated in cell signaling, organization of the cytoskeleton, and control of protein biosynthesis under specific cellular circumstances. Clearly, PKPs are more than just cell adhesion proteins. In this paper we will give an overview of our current knowledge on the very distinct roles of plakophilins in the cell.
Figures


References
-
- Cheng X, Den Z, Koch PJ. Desmosomal cell adhesion in mammalian development. European Journal of Cell Biology. 2005;84(2-3):215–223. - PubMed
-
- Holthöfer B, Windoffer R, Troyanovsky S, Leube RE. Structure and function of desmosomes. International Review of Cytology. 2007;264:65–163. - PubMed
-
- Schmidt A, Koch PJ. Desmosomes in development and disease. In: LaFlamme SE, Kowalczyk AP, editors. Cell Junctions: Adhesion, Development, and Disease. Weinheim, Germany: Wiley-VCH; 2008. pp. 235–249.
-
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. Journal of Investigative Dermatology. 2007;127(11):2499–2515. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

