Mammalian sleep dynamics: how diverse features arise from a common physiological framework
- PMID: 20585613
- PMCID: PMC2891699
- DOI: 10.1371/journal.pcbi.1000826
Mammalian sleep dynamics: how diverse features arise from a common physiological framework
Abstract
Mammalian sleep varies widely, ranging from frequent napping in rodents to consolidated blocks in primates and unihemispheric sleep in cetaceans. In humans, rats, mice and cats, sleep patterns are orchestrated by homeostatic and circadian drives to the sleep-wake switch, but it is not known whether this system is ubiquitous among mammals. Here, changes of just two parameters in a recent quantitative model of this switch are shown to reproduce typical sleep patterns for 17 species across 7 orders. Furthermore, the parameter variations are found to be consistent with the assumptions that homeostatic production and clearance scale as brain volume and surface area, respectively. Modeling an additional inhibitory connection between sleep-active neuronal populations on opposite sides of the brain generates unihemispheric sleep, providing a testable hypothetical mechanism for this poorly understood phenomenon. Neuromodulation of this connection alone is shown to account for the ability of fur seals to transition between bihemispheric sleep on land and unihemispheric sleep in water. Determining what aspects of mammalian sleep patterns can be explained within a single framework, and are thus universal, is essential to understanding the evolution and function of mammalian sleep. This is the first demonstration of a single model reproducing sleep patterns for multiple different species. These wide-ranging findings suggest that the core physiological mechanisms controlling sleep are common to many mammalian orders, with slight evolutionary modifications accounting for interspecies differences.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

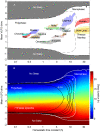

 ,
,  h). Elephant: (C) data from , (D) model (
h). Elephant: (C) data from , (D) model ( ,
,  h). Opossum: (E) data from , (F) model (
h). Opossum: (E) data from , (F) model ( ,
,  h). Noise is added to the model to make sleep patterns less regular (see Methods for numerical details).
h). Noise is added to the model to make sleep patterns less regular (see Methods for numerical details).
 ), corresponding to a power law with exponent 0.29±0.10 (Mean±S.D. calculated using bootstrapping), and for primates (dashed,
), corresponding to a power law with exponent 0.29±0.10 (Mean±S.D. calculated using bootstrapping), and for primates (dashed,  ) with exponent 0.01±0.26. A linear fit to all species (
) with exponent 0.01±0.26. A linear fit to all species ( ) yields an exponent of 0.28±0.12.
) yields an exponent of 0.28±0.12.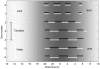
 during the transition period on days 2–4.
during the transition period on days 2–4.Similar articles
-
Physiologically based quantitative modeling of unihemispheric sleep.J Theor Biol. 2012 Dec 7;314:109-19. doi: 10.1016/j.jtbi.2012.08.031. Epub 2012 Sep 1. J Theor Biol. 2012. PMID: 22960411
-
Common scale-invariant patterns of sleep-wake transitions across mammalian species.Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17545-8. doi: 10.1073/pnas.0408242101. Epub 2004 Dec 6. Proc Natl Acad Sci U S A. 2004. PMID: 15583127 Free PMC article.
-
Fur seals display a strong drive for bilateral slow-wave sleep while on land.J Neurosci. 2008 Nov 26;28(48):12614-21. doi: 10.1523/JNEUROSCI.2306-08.2008. J Neurosci. 2008. PMID: 19036955 Free PMC article.
-
Unihemispheric sleep and asymmetrical sleep: behavioral, neurophysiological, and functional perspectives.Nat Sci Sleep. 2016 Jul 12;8:221-38. doi: 10.2147/NSS.S71970. eCollection 2016. Nat Sci Sleep. 2016. PMID: 27471418 Free PMC article. Review.
-
Behavioral, neurophysiological and evolutionary perspectives on unihemispheric sleep.Neurosci Biobehav Rev. 2000 Dec;24(8):817-42. doi: 10.1016/s0149-7634(00)00039-7. Neurosci Biobehav Rev. 2000. PMID: 11118608 Review.
Cited by
-
Sleep Modelling across Physiological Levels.Clocks Sleep. 2019 Mar 4;1(1):166-184. doi: 10.3390/clockssleep1010015. eCollection 2019 Mar. Clocks Sleep. 2019. PMID: 33089162 Free PMC article. Review.
-
Mathematical models for sleep-wake dynamics: comparison of the two-process model and a mutual inhibition neuronal model.PLoS One. 2014 Aug 1;9(8):e103877. doi: 10.1371/journal.pone.0103877. eCollection 2014. PLoS One. 2014. PMID: 25084361 Free PMC article.
-
Mapping the physiological changes in sleep regulation across infancy and young childhood.PLoS Comput Biol. 2024 Oct 21;20(10):e1012541. doi: 10.1371/journal.pcbi.1012541. eCollection 2024 Oct. PLoS Comput Biol. 2024. PMID: 39432549 Free PMC article.
-
A physiologically based model of orexinergic stabilization of sleep and wake.PLoS One. 2014 Mar 20;9(3):e91982. doi: 10.1371/journal.pone.0091982. eCollection 2014. PLoS One. 2014. PMID: 24651580 Free PMC article.
-
A comparative study of sleep and diurnal patterns in house mouse (Mus musculus) and Spiny mouse (Acomys cahirinus).Sci Rep. 2020 Jul 2;10(1):10944. doi: 10.1038/s41598-020-67859-w. Sci Rep. 2020. PMID: 32616800 Free PMC article.
References
-
- Campbell SS, Tobler I. Animal sleep: A review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. - PubMed
-
- Tobler I. Is sleep fundamentally different between mammalian species? Behav Brain Res. 1995;69:35–41. - PubMed
-
- Mukhametov LM. Unihemispheric slow-wave sleep in the Amazonian dolphin, Inia geoffrensis. Neurosci Lett. 1987;79:128–132. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

