Drosophila genome-wide RNAi screen identifies multiple regulators of HIF-dependent transcription in hypoxia
- PMID: 20585616
- PMCID: PMC2891703
- DOI: 10.1371/journal.pgen.1000994
Drosophila genome-wide RNAi screen identifies multiple regulators of HIF-dependent transcription in hypoxia
Abstract
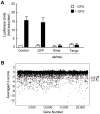
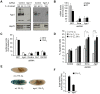
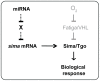
Hypoxia-inducible factors (HIFs) are a family of evolutionary conserved alpha-beta heterodimeric transcription factors that induce a wide range of genes in response to low oxygen tension. Molecular mechanisms that mediate oxygen-dependent HIF regulation operate at the level of the alpha subunit, controlling protein stability, subcellular localization, and transcriptional coactivator recruitment. We have conducted an unbiased genome-wide RNA interference (RNAi) screen in Drosophila cells aimed to the identification of genes required for HIF activity. After 3 rounds of selection, 30 genes emerged as critical HIF regulators in hypoxia, most of which had not been previously associated with HIF biology. The list of genes includes components of chromatin remodeling complexes, transcription elongation factors, and translational regulators. One remarkable hit was the argonaute 1 (ago1) gene, a central element of the microRNA (miRNA) translational silencing machinery. Further studies confirmed the physiological role of the miRNA machinery in HIF-dependent transcription. This study reveals the occurrence of novel mechanisms of HIF regulation, which might contribute to developing novel strategies for therapeutic intervention of HIF-related pathologies, including heart attack, cancer, and stroke.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
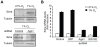

References
-
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. - PubMed
-
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. - PubMed
-
- Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277:26351–26355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

