Biodistribution and dosimetry of (18)F-EF5 in cancer patients with preliminary comparison of (18)F-EF5 uptake versus EF5 binding in human glioblastoma
- PMID: 20585774
- PMCID: PMC2948639
- DOI: 10.1007/s00259-010-1517-y
Biodistribution and dosimetry of (18)F-EF5 in cancer patients with preliminary comparison of (18)F-EF5 uptake versus EF5 binding in human glioblastoma
Abstract
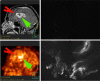
Purpose: The primary purpose of this study was to assess the biodistribution and radiation dose resulting from administration of (18)F-EF5, a lipophilic 2-nitroimidazole hypoxia marker in ten cancer patients. For three of these patients (with glioblastoma) unlabeled EF5 was additionally administered to allow the comparative assessment of (18)F-EF5 tumor uptake with EF5 binding, the latter measured in tumor biopsies by fluorescent anti-EF5 monoclonal antibodies.
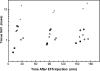
Methods: (18)F-EF5 was synthesized by electrophilic addition of (18)F(2) gas, made by deuteron bombardment of a neon/fluorine mixture in a high-pressure gas target, to an allyl precursor in trifluoroacetic acid at 0° then purified and administered by intravenous bolus. Three whole-body images were collected for each of ten patients using an Allegro (Philips) scanner. Gamma counts were determined in blood, drawn during each image, and urine, pooled as a single sample. PET images were analyzed to determine radiotracer uptake in several tissues and the resulting radiation dose calculated using OLINDA software and standard phantom. For three patients, 21 mg/kg unlabeled EF5 was administered after the PET scans, and tissue samples obtained the next day at surgery to determine EF5 binding using immunohistochemistry techniques (IHC).
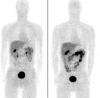
Results: EF5 distributes evenly throughout soft tissue within minutes of injection. Its concentration in blood over the typical time frame of the study (∼3.5 h) was nearly constant, consistent with a previously determined EF5 plasma half-life of ∼13 h. Elimination was primarily via urine and bile. Radiation exposure from labeled EF5 is similar to other (18)F-labeled imaging agents (e.g., FDG and FMISO). In a de novo glioblastoma multiforme patient, focal uptake of (18)F-EF5 was confirmed by IHC.
Conclusion: These results confirm predictions of biodistribution and safety based on EF5's characteristics (high biological stability, high lipophilicity). EF5 is a novel hypoxia marker with unique pharmacological characteristics allowing both noninvasive and invasive measurements.
Figures


References
-
- Evans SM, Koch CJ. Prognostic significance of tumor oxygenation in humans. Cancer Lett. 2003;195:1–16. - PubMed
-
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. - PubMed
-
- Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KSC, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. - PubMed
-
- Wilson GD. Hypoxia and prognosis: the oxygen tension mounts. Front Biosci. 2007;12:3502–18. - PubMed
-
- Koch CJ. Competition between radiation protectors and radiation sensitizers in mammalian cells. In: Nygaard OF, Simic MG, editors. Radioprotectors and anticarcinogens. Academic; New York: 1983. pp. 275–96.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

