Elucidation of the functional metal binding profile of a Cd(II)/Pb(II) sensor CmtR(Sc) from Streptomyces coelicolor
- PMID: 20586430
- PMCID: PMC2925124
- DOI: 10.1021/bi100490u
Elucidation of the functional metal binding profile of a Cd(II)/Pb(II) sensor CmtR(Sc) from Streptomyces coelicolor
Abstract
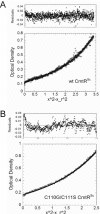
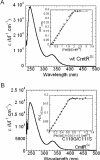
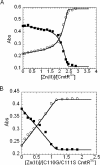
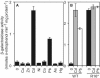
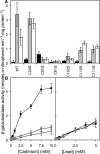
Metal homeostasis and resistance in bacteria is maintained by a panel of metal-sensing transcriptional regulators that collectively control transition metal availability and mediate resistance to heavy metal xenobiotics, including As(III), Cd(II), Pb(II), and Hg(II). The ArsR family constitutes a superfamily of metal sensors that appear to conform to the same winged helical, homodimeric fold, that collectively "sense" a wide array of beneficial metal ions and heavy metal pollutants. The genomes of many actinomycetes, including the soil dwelling bacterium Streptomyces coelicolor and the human pathogen Mycobacterium tuberculosis, encode over ten ArsR family regulators, most of unknown function. Here, we present the characterization of a homologue of M. tuberculosis CmtR (CmtR(Mtb)) from S. coelicolor, denoted CmtR(Sc). We show that CmtR(Sc), in contrast to CmtR(Mtb), binds two monomer mol equivalents of Pb(II) or Cd(II) to form two pairs of sulfur-rich coordination complexes per dimer. Metal site 1 conforms exactly to the alpha4C site previously characterized in CmtR(Mtb) while metal site 2 is coordinated by a C-terminal vicinal thiolate pair, Cys110 and Cys111. Biological assays reveal that only Cd(II) and, to a lesser extent, Pb(II) mediate transcriptional derepression in the heterologous host Mycobacterium smegmatis in a way that requires metal site 1. In contrast, mutagenesis of metal site 2 ligands Cys110 or Cys111 significantly reduces Cd(II) responsiveness, with no detectable effect on Pb(II) sensing. The implications of these findings on the ability to predict metal specificity and function from metal-site signatures in the primary structure of ArsR family proteins are discussed.
Figures



Similar articles
-
Structural and functional characterization of Mycobacterium tuberculosis CmtR, a PbII/CdII-sensing SmtB/ArsR metalloregulatory repressor.Biochemistry. 2005 Jun 28;44(25):8976-88. doi: 10.1021/bi050094v. Biochemistry. 2005. PMID: 15966722
-
A cadmium-lead-sensing ArsR-SmtB repressor with novel sensory sites. Complementary metal discrimination by NmtR AND CmtR in a common cytosol.J Biol Chem. 2003 Nov 7;278(45):44560-6. doi: 10.1074/jbc.M307877200. Epub 2003 Aug 25. J Biol Chem. 2003. PMID: 12939264
-
Elucidation of primary (alpha(3)N) and vestigial (alpha(5)) heavy metal-binding sites in Staphylococcus aureus pI258 CadC: evolutionary implications for metal ion selectivity of ArsR/SmtB metal sensor proteins.J Mol Biol. 2002 Jun 7;319(3):685-701. doi: 10.1016/S0022-2836(02)00299-1. J Mol Biol. 2002. PMID: 12054863
-
The SmtB/ArsR family of metalloregulatory transcriptional repressors: Structural insights into prokaryotic metal resistance.FEMS Microbiol Rev. 2003 Jun;27(2-3):131-43. doi: 10.1016/S0168-6445(03)00054-8. FEMS Microbiol Rev. 2003. PMID: 12829264 Review.
-
Structural determinants of metal selectivity in prokaryotic metal-responsive transcriptional regulators.Biometals. 2005 Aug;18(4):413-28. doi: 10.1007/s10534-005-3716-8. Biometals. 2005. PMID: 16158234 Review.
Cited by
-
Allosteric inhibition of a zinc-sensing transcriptional repressor: insights into the arsenic repressor (ArsR) family.J Mol Biol. 2013 Apr 12;425(7):1143-57. doi: 10.1016/j.jmb.2013.01.018. Epub 2013 Jan 23. J Mol Biol. 2013. PMID: 23353829 Free PMC article.
-
Bacterial Metallostasis: Metal Sensing, Metalloproteome Remodeling, and Metal Trafficking.Chem Rev. 2024 Dec 25;124(24):13574-13659. doi: 10.1021/acs.chemrev.4c00264. Epub 2024 Dec 10. Chem Rev. 2024. PMID: 39658019 Free PMC article. Review.
-
Recent advances in bacterial biosensing and bioremediation of cadmium pollution: a mini-review.World J Microbiol Biotechnol. 2021 Dec 1;38(1):9. doi: 10.1007/s11274-021-03198-w. World J Microbiol Biotechnol. 2021. PMID: 34850291 Review.
-
Antimicrobial Nanomaterials: Why Evolution Matters.Nanomaterials (Basel). 2017 Sep 21;7(10):283. doi: 10.3390/nano7100283. Nanomaterials (Basel). 2017. PMID: 28934114 Free PMC article.
-
MALDI-TOF-MS and 16S rRNA characterization of lead tolerant metallophile bacteria isolated from saffron soils of Kashmir for their sequestration potential.Saudi J Biol Sci. 2020 Aug;27(8):2047-2053. doi: 10.1016/j.sjbs.2020.04.021. Epub 2020 Apr 20. Saudi J Biol Sci. 2020. PMID: 32714029 Free PMC article.
References
-
- Nies DH. Heavy metal-resistant bacteria as extremophiles: molecular physiology and biotechnological use of Ralstonia sp. CH34. Extremophiles. 2000;4:77–82. - PubMed
-
- Payne JC, ter Horst MA, Godwin HA. Lead fingers: Pb2+ binding to structural zinc-binding domains determined directly by monitoring lead-thiolate charge-transfer bands. J. Am. Chem. Soc. 1999;121:6850–6855.
-
- Warren MJ, Cooper JB, Wood SP, Shoolingin-Jordan PM. Lead poisoning, haem synthesis and 5-aminolaevulinic acid dehydratase. Trends Biochem Sci. 1998;23:217–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

