Development of an enantioselective route toward the Lycopodium alkaloids: total synthesis of lycopodine
- PMID: 20586477
- PMCID: PMC2943527
- DOI: 10.1021/jo100916x
Development of an enantioselective route toward the Lycopodium alkaloids: total synthesis of lycopodine
Abstract
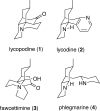
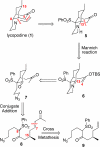
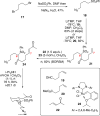
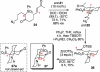
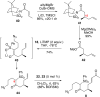
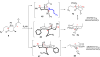
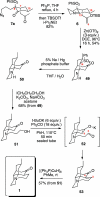
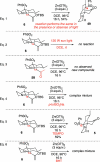
Synthesis of a C(15)-desmethyl tricycle core of lycopodine has been accomplished. Key steps in the synthetic sequence include organocatalytic, intramolecular Michael addition of a keto sulfone and a tandem 1,3-sulfonyl shift/Mannich cyclization to construct the tricyclic core ring system. Synthetic work toward this natural product family led to the development of N-(p-dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide, an organocatalyst which facilitates enantioselective, intramolecular Michael additions. A detailed mechanistic discussion is provided for both the intramolecular Michael addition and the sulfone rearrangement. Finally, the application of these discoveries to the enantioselective total synthesis of alkaloid lycopodine is described.
Figures
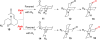
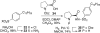
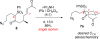
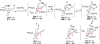
Similar articles
-
Toward a unified approach for the lycopodines: synthesis of 10-hydroxylycopodine, deacetylpaniculine, and paniculine.Org Lett. 2013 Feb 15;15(4):736-9. doi: 10.1021/ol303272w. Epub 2013 Feb 5. Org Lett. 2013. PMID: 23384410 Free PMC article.
-
Enantioselective total synthesis of lycopodine.J Am Chem Soc. 2008 Jul 23;130(29):9238-9. doi: 10.1021/ja803613w. Epub 2008 Jun 27. J Am Chem Soc. 2008. PMID: 18582046 Free PMC article.
-
Unified Synthesis of 10-Oxygenated Lycopodium Alkaloids: Impact of C10-Stereochemistry on Reactivity.J Org Chem. 2016 Jul 15;81(14):5963-80. doi: 10.1021/acs.joc.6b00900. Epub 2016 Jun 29. J Org Chem. 2016. PMID: 27353498
-
Total Syntheses of Lycopodium and Monoterpenoid Indole Alkaloids Based on Biosynthesis-Inspired Strategies.Chem Pharm Bull (Tokyo). 2020;68(2):103-116. doi: 10.1248/cpb.c19-00872. Chem Pharm Bull (Tokyo). 2020. PMID: 32009077 Review.
-
Strategies and Efforts towards the Total Synthesis of Palhinine Alkaloids.Molecules. 2020 Sep 14;25(18):4211. doi: 10.3390/molecules25184211. Molecules. 2020. PMID: 32937904 Free PMC article. Review.
Cited by
-
Recent Advances in the Chemistry of Saturated Annulated Nitrogen-Containing Polycyclic Compounds.Int J Mol Sci. 2022 Dec 7;23(24):15484. doi: 10.3390/ijms232415484. Int J Mol Sci. 2022. PMID: 36555128 Free PMC article. Review.
-
Proline Sulfonamide-Based Organocatalysis: Better Late than Never.Synlett. 2010 Sep 1;2010(19):2827-2838. doi: 10.1055/s-0030-1259020. Synlett. 2010. PMID: 21461132 Free PMC article.
-
Toward a unified approach for the lycopodines: synthesis of 10-hydroxylycopodine, deacetylpaniculine, and paniculine.Org Lett. 2013 Feb 15;15(4):736-9. doi: 10.1021/ol303272w. Epub 2013 Feb 5. Org Lett. 2013. PMID: 23384410 Free PMC article.
-
Total synthesis of the Lycopodium alkaloid serratezomine A using free radical-mediated vinyl amination to prepare a β-stannyl enamine linchpin.J Org Chem. 2013 Feb 1;78(3):822-43. doi: 10.1021/jo302333s. Epub 2012 Dec 28. J Org Chem. 2013. PMID: 23273261 Free PMC article.
-
Highly stereoselective and scalable anti-aldol reactions using N-(p-dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide: scope and origins of stereoselectivities.J Org Chem. 2010 Nov 5;75(21):7279-90. doi: 10.1021/jo1015008. J Org Chem. 2010. PMID: 20932013 Free PMC article.
References
-
- Bödeker K. Justus Liebigs Ann. Chem. 1881;208:363–367.
-
- Achmatowicsz O, Uzieblo W. Rocz. Chem. 1938;18:88–95.
-
- Ayer WA, Iverach GG. Tetrahedron Lett. 1962;3:87–92. - PubMed
-
- Rogers D, Quick A, Hague M. Acta Cryst. 1974;B30:552–553.
- Hague M, Rogers D. J. Chem. Soc., Perkin Trans. 2. 1975:93–98.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

