Development of an enantioselective route toward the Lycopodium alkaloids: total synthesis of lycopodine
- PMID: 20586477
- PMCID: PMC2943527
- DOI: 10.1021/jo100916x
Development of an enantioselective route toward the Lycopodium alkaloids: total synthesis of lycopodine
Abstract
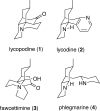
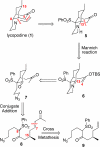
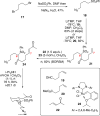
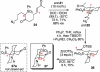
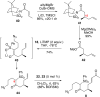
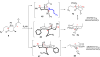
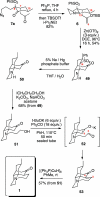
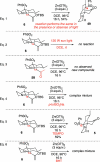
Synthesis of a C(15)-desmethyl tricycle core of lycopodine has been accomplished. Key steps in the synthetic sequence include organocatalytic, intramolecular Michael addition of a keto sulfone and a tandem 1,3-sulfonyl shift/Mannich cyclization to construct the tricyclic core ring system. Synthetic work toward this natural product family led to the development of N-(p-dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide, an organocatalyst which facilitates enantioselective, intramolecular Michael additions. A detailed mechanistic discussion is provided for both the intramolecular Michael addition and the sulfone rearrangement. Finally, the application of these discoveries to the enantioselective total synthesis of alkaloid lycopodine is described.
Figures
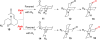
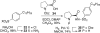
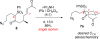
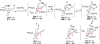
References
-
- Bödeker K. Justus Liebigs Ann. Chem. 1881;208:363–367.
-
- Achmatowicsz O, Uzieblo W. Rocz. Chem. 1938;18:88–95.
-
- Ayer WA, Iverach GG. Tetrahedron Lett. 1962;3:87–92. - PubMed
-
- Rogers D, Quick A, Hague M. Acta Cryst. 1974;B30:552–553.
- Hague M, Rogers D. J. Chem. Soc., Perkin Trans. 2. 1975:93–98.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

