Generation of easily accessible human kidney tubules on two-dimensional surfaces in vitro
- PMID: 20586829
- PMCID: PMC4373329
- DOI: 10.1111/j.1582-4934.2010.01113.x
Generation of easily accessible human kidney tubules on two-dimensional surfaces in vitro
Abstract
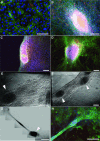
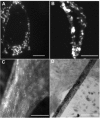
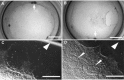
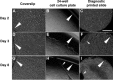
The generation of tissue-like structures in vitro is of major interest for various fields of research including in vitro toxicology, regenerative therapies and tissue engineering. Usually 3D matrices are used to engineer tissue-like structures in vitro, and for the generation of kidney tubules, 3D gels are employed. Kidney tubules embedded within 3D gels are difficult to access for manipulations and imaging. Here we show how large and functional human kidney tubules can be generated in vitro on 2D surfaces, without the use of 3D matrices. The mechanism used by human primary renal proximal tubule cells for tubulogenesis on 2D surfaces appears to be distinct from the mechanism employed in 3D gels, and tubulogenesis on 2D surfaces involves interactions between epithelial and mesenchymal cells. The process is induced by transforming growth factor-β(1), and enhanced by a 3D substrate architecture. However, after triggering the process, the formation of renal tubules occurs with remarkable independence from the substrate architecture. Human proximal tubules generated on 2D surfaces typically have a length of several millimetres, and are easily accessible for manipulations and imaging, which makes them attractive for basic research and in vitro nephrotoxicology. The experimental system described also allows for in vitro studies on how primary human kidney cells regenerate renal structures after organ disruption. The finding that human kidney cells organize tissue-like structures independently from the substrate architecture has important consequences for kidney tissue engineering, and it will be important, for instance, to inhibit the process of tubulogenesis on 2D surfaces in bioartificial kidneys.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures




References
-
- Dressler G. Tubulogenesis in the developing mammalian kidney. Trends Cell Biol. 2002;12:390–5. - PubMed
-
- Vainio S, Lin Y. Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet. 2002;3:533–43. - PubMed
-
- Han HJ, Sigurdson WJ, Nickerson PA, et al. Both mitogen activated protein kinase and the mammalian target of rapamycin modulate the development of functional renal proximal tubules in matrigel. J Cell Sci. 2004;117:1821–33. - PubMed
-
- Humes HD, Krauss JC, Cieslinski DA, et al. Tubulogenesis from isolated single cells of adult mammalian kidney: clonal analysis with a recombinant retrovirus. Am J Physiol. 1996;271:F42–9. - PubMed
-
- Karihaloo A, Nickel C, Cantley LG. Signals which build a tubule. Nephron Exp Nephrol. 2005;100:e40–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

