Model thrombi formed under flow reveal the role of factor XIII-mediated cross-linking in resistance to fibrinolysis
- PMID: 20586921
- PMCID: PMC3071935
- DOI: 10.1111/j.1538-7836.2010.03963.x
Model thrombi formed under flow reveal the role of factor XIII-mediated cross-linking in resistance to fibrinolysis
Abstract
Background: Activated factor XIII (FXIIIa), a transglutaminase, introduces fibrin-fibrin and fibrin-inhibitor cross-links, resulting in more mechanically stable clots. The impact of cross-linking on resistance to fibrinolysis has proved challenging to evaluate quantitatively.
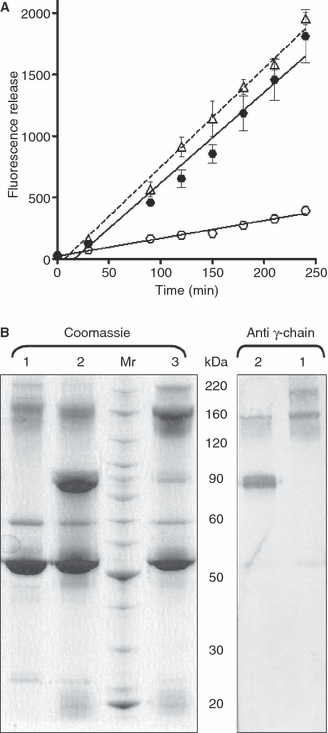
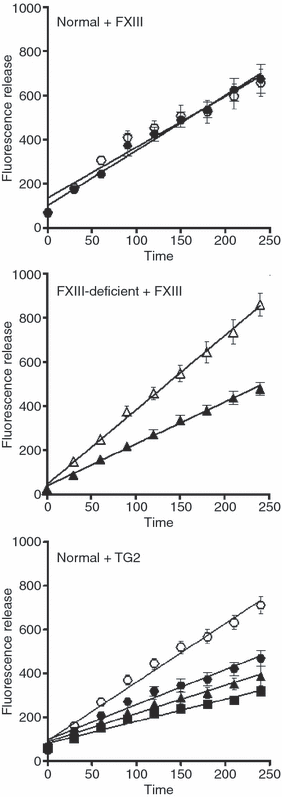
Methods: We used a whole blood model thrombus system to characterize the role of cross-linking in resistance to fibrinolytic degradation. Model thrombi, which mimic arterial thrombi formed in vivo, were prepared with incorporated fluorescently labeled fibrinogen, in order to allow quantification of fibrinolysis as released fluorescence units per minute.
Results: A site-specific inhibitor of transglutaminases, added to blood from normal donors, yielded model thrombi that lysed more easily, either spontaneously or by plasminogen activators. This was observed both in the cell/platelet-rich head and fibrin-rich tail. Model thrombi from an FXIII-deficient patient lysed more quickly than normal thrombi; replacement therapy with FXIII concentrate normalized lysis. In vitro addition of purified FXIII to the patient's preprophylaxis blood, but not to normal control blood, resulted in more stable thrombi, indicating no further efficacy of supraphysiologic FXIII. However, addition of tissue transglutaminase, which is synthesized by endothelial cells, generated thrombi that were more resistant to fibrinolysis; this may stabilize mural thrombi in vivo.
Conclusions: Model thrombi formed under flow, even those prepared as plasma 'thrombi', reveal the effect of FXIII on fibrinolysis. Although very low levels of FXIII are known to produce mechanical clot stability, and to achieve γ-dimerization, they appear to be suboptimal in conferring full resistance to fibrinolysis.
© 2010 International Society on Thrombosis and Haemostasis.
Figures



References
-
- Anwar R, Miloszewski KJ. Factor XIII deficiency. Br J Haematol. 1999;107:468–84. - PubMed
-
- Koseki-Kuno S, Yamakawa M, Dickneite G, Ichinose A. Factor XIII A subunit-deficient mice developed severe uterine bleeding events and subsequent spontaneous miscarriages. Blood. 2003;102:4410–12. - PubMed
-
- Ichinose A. Physiopathology and regulation of factor XIII. Thromb Haemost. 2001;86:57–65. - PubMed
-
- Lorand L. Sol Sherry lecture in thrombosis: research on clot stabilization provides clues for improving thrombolytic therapies. Arterioscler Thromb Vasc Biol. 2000;20:2–9. - PubMed
-
- Naski MC, Lorand L, Shafer JA. Characterization of the kinetic pathway for fibrin promotion of alpha-thrombin-catalyzed activation of plasma factor XIII. Biochemistry. 1991;30:934–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

