Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: systematic review
- PMID: 20586956
- PMCID: PMC2948795
- DOI: 10.1111/j.1365-3156.2010.02508.x
Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: systematic review
Abstract
Objectives: To estimate the proportion of all-cause adult patient attrition from antiretroviral therapy (ART) programs in service delivery settings in sub-Saharan Africa through 36 months on treatment.
Methods: We identified cohorts within Ovid Medline, ISI Web of Knowledge, Cochrane Database of Systematic Reviews and four conference abstract archives. We summarized retention rates from studies describing observational cohorts from sub-Saharan Africa reporting on adult HIV 1- infected patients initiating first-line three-drug ART. We estimated all-cause attrition rates for 6, 12, 18, 24, or 36 months after ART initiation including patients who died or were lost to follow-up (as defined by the author), but excluding transferred patients.
Results: We analysed 33 sources describing 39 cohorts and 226 307 patients. Patients were more likely to be female (median 65%) and had a median age at initiation of 37 (range 34-40). Median starting CD4 count was 109 cells/mm(3). Loss to follow-up was the most common cause of attrition (59%), followed by death (41%). Median attrition at 12, 24 and 36 months was 22.6% (range 7%-45%), 25% (range 11%-32%) and 29.5% (range 13%-36.1%) respectively. After pooling data in a random-effects meta-analysis, retention declined from 86.1% at 6 months to 80.2% at 12 months, 76.8% at 24 months and 72.3% at 36 months. Adjusting for variable follow-up time in a sensitivity analysis, 24 month retention was 70.0% (range: 66.7%-73.3%), while 36 month retention was 64.6% (range: 57.5%-72.1%).
Conclusions: Our findings document the difficulties in retaining patients in care for lifelong treatment, and the progress being made in raising overall retention rates.
Figures
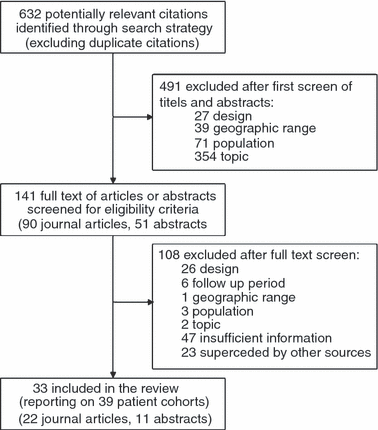


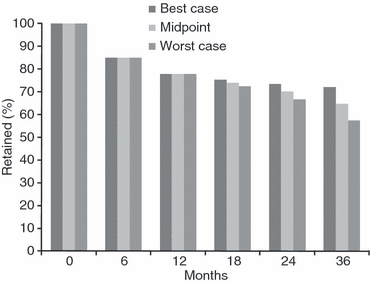
Comment in
-
Defaulting from antiretroviral treatment programmes in sub-Saharan Africa: a problem of definition.Trop Med Int Health. 2011 Mar;16(3):390-1; author reply 392. doi: 10.1111/j.1365-3156.2010.02693.x. Epub 2010 Dec 8. Trop Med Int Health. 2011. PMID: 21143352 No abstract available.
References
-
- Anglaret X, Toure S, Gourvellec G, et al. Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from Sub-Saharan Africa. Journal of Acquired Immune Deficiency Syndromes. 2004;35:320–323. - PubMed
-
- Auld AF. Treatment Outcomes of HIV-Infected Adults Enrolled in the National Antiretroviral Therapy Program -Mozambique, 2004-2007. Namibia: HIV/AIDS Implementers' Meeting Windhoek; 2009. (Abstract 1608)
-
- Badri M, Cleary S, Maartens G, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antiviral Therapy. 2006;11:63–72. - PubMed
-
- Bajunirwe F, Arts EJ, Tisch DJ, Debanne SM, Sethi AK. Survival, Adherence to Care and Antiretroviral Treatment (ART) Among HIV-Infected Adults in Rural Western Uganda. 2007. 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention WEPEB049.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

