In vivo action of trefoil factor 2 (TFF2) to speed gastric repair is independent of cyclooxygenase
- PMID: 20587547
- PMCID: PMC3686652
- DOI: 10.1136/gut.2009.205625
In vivo action of trefoil factor 2 (TFF2) to speed gastric repair is independent of cyclooxygenase
Abstract
Objective: Trefoil factor (TFF) peptides are expressed in gastric tissues, where they are part of the epithelial defences. To complement previous in vitro work, the goal of the present study was to examine directly if TFF2 was essential for gastric restitution in vivo during the recovery from microscopic damage.
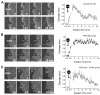
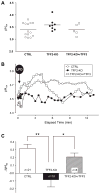
Design: TFF2 mutant (KO) mice were examined to study the epithelial repair process in vivo after laser-induced photodamage (LPD). Using two-photon laser energy absorption (710 nm), LPD was imposed on an approximately 3-5 cell region of surface epithelium in anaesthetised mouse stomach. Responses to damage were evaluated during confocal time-lapse microscopy; including area of damage and the extracellular pH adjacent to the damaged surface (Cl-NERF pH sensor).
Results: In control (TFF2+/+ and TFF2+/-) mice, damaged cells were exfoliated and the damaged epithelium was repaired by indomethacin. The resting surface pH was similar between control and TFF2-KO animals, but the post-LPD alkalisation of surface pH observed in control mice (pH 0.3 + or - 0.05, n=21) was attenuated in the TFF2-KO stomach (pH -0.08 + or - 0.09, n=18). Recobinant rat TFF3 partially rescued the attenuated surface pH change in TFF2-KO stomach, in the presence or absence of indomethacin.
Conclusions: In the gastric epithelium in vivo, TFFs promote epithelial restitution via a mechanism that does not require cyclooxygenase activation. A novel role for TFFs to affect gastric surface pH is observed.
Conflict of interest statement
Figures



Similar articles
-
Trefoil factor 2 requires Na/H exchanger 2 activity to enhance mouse gastric epithelial repair.J Biol Chem. 2011 Nov 4;286(44):38375-38382. doi: 10.1074/jbc.M111.268219. Epub 2011 Sep 7. J Biol Chem. 2011. PMID: 21900251 Free PMC article.
-
Trefoil factor 2 activation of CXCR4 requires calcium mobilization to drive epithelial repair in gastric organoids.J Physiol. 2019 May;597(10):2673-2690. doi: 10.1113/JP277259. Epub 2019 Apr 14. J Physiol. 2019. PMID: 30912855 Free PMC article.
-
TFF2 deficiency exacerbates weight loss and alters immune cell and cytokine profiles in DSS colitis, and this cannot be rescued by wild-type bone marrow.Am J Physiol Gastrointest Liver Physiol. 2015 Jan 1;308(1):G12-24. doi: 10.1152/ajpgi.00172.2014. Epub 2014 Oct 16. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25324506 Free PMC article.
-
Trefoil factor family 2 deficiency and immune response.Cell Mol Life Sci. 2005 Dec;62(24):2947-55. doi: 10.1007/s00018-005-5483-7. Cell Mol Life Sci. 2005. PMID: 16374583 Free PMC article. Review.
-
Trefoil factors and human gastric cancer (review).Int J Mol Med. 2003 Jul;12(1):3-9. Int J Mol Med. 2003. PMID: 12792801 Review.
Cited by
-
Trefoil Factor Peptides and Gastrointestinal Function.Annu Rev Physiol. 2017 Feb 10;79:357-380. doi: 10.1146/annurev-physiol-021115-105447. Epub 2016 Dec 15. Annu Rev Physiol. 2017. PMID: 27992733 Free PMC article. Review.
-
Within-host evolution of Helicobacter pylori shaped by niche-specific adaptation, intragastric migrations and selective sweeps.Nat Commun. 2019 May 22;10(1):2273. doi: 10.1038/s41467-019-10050-1. Nat Commun. 2019. PMID: 31118420 Free PMC article.
-
Contribution of Wound-Associated Cells and Mediators in Orchestrating Gastrointestinal Mucosal Wound Repair.Annu Rev Physiol. 2019 Feb 10;81:189-209. doi: 10.1146/annurev-physiol-020518-114504. Epub 2018 Oct 24. Annu Rev Physiol. 2019. PMID: 30354933 Free PMC article. Review.
-
Trefoil factor 2 requires Na/H exchanger 2 activity to enhance mouse gastric epithelial repair.J Biol Chem. 2011 Nov 4;286(44):38375-38382. doi: 10.1074/jbc.M111.268219. Epub 2011 Sep 7. J Biol Chem. 2011. PMID: 21900251 Free PMC article.
-
Loss of Trefoil Factor 2 Sensitizes Rat Pups to Systemic Infection with the Neonatal Pathogen Escherichia coli K1.Infect Immun. 2019 Apr 23;87(5):e00878-18. doi: 10.1128/IAI.00878-18. Print 2019 Mar. Infect Immun. 2019. PMID: 30833331 Free PMC article.
References
-
- Kvietys PR, Specian RD, Grisham MB, et al. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol. 1991;261:G384–91. - PubMed
-
- Lacy ER. Epithelial restitution in the gastrointestinal tract. J Clin Gastroenterol. 1988;10(Suppl 1):S72–7. - PubMed
-
- Lacy ER, Ito S. Rapid epithelial restitution of the rat gastric mucosa after ethanol injury. Lab Invest. 1984;51:573–83. - PubMed
-
- Silen W, Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu Rev Physiol. 1985;47:217–29. - PubMed
-
- Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
