Molecular characterization of EG-VEGF-mediated angiogenesis: differential effects on microvascular and macrovascular endothelial cells
- PMID: 20587779
- PMCID: PMC2921113
- DOI: 10.1091/mbc.E10-01-0059
Molecular characterization of EG-VEGF-mediated angiogenesis: differential effects on microvascular and macrovascular endothelial cells
Abstract
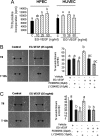
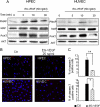
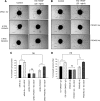
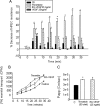
Endocrine gland derived vascular endothelial growth factor (EG-VEGF) also called prokineticin (PK1), has been identified and linked to several biological processes including angiogenesis. EG-VEGF is abundantly expressed in the highest vascularized organ, the human placenta. Here we characterized its angiogenic effect using different experimental procedures. Immunohistochemistry was used to localize EG-VEGF receptors (PROKR1 and PROKR2) in placental and umbilical cord tissue. Primary microvascular placental endothelial cell (HPEC) and umbilical vein-derived macrovascular EC (HUVEC) were used to assess its effects on proliferation, migration, cell survival, pseudovascular organization, spheroid sprouting, permeability and paracellular transport. siRNA and neutralizing antibody strategies were used to differentiate PROKR1- from PROKR2-mediated effects. Our results show that 1) HPEC and HUVEC express both types of receptors 2) EG-VEGF stimulates HPEC's proliferation, migration and survival, but increases only survival in HUVECs. and 3) EG-VEGF was more potent than VEGF in stimulating HPEC sprout formation, pseudovascular organization, and it significantly increases HPEC permeability and paracellular transport. More importantly, we demonstrated that PROKR1 mediates EG-VEGF angiogenic effects, whereas PROKR2 mediates cellular permeability. Altogether, these data characterized angiogenic processes mediated by EG-VEGF, depicted a new angiogenic factor in the placenta, and suggest a novel view of the regulation of angiogenesis in placental pathologies.
Figures




References
-
- Burton G. J., Jauniaux E. Sonographic, stereological and Doppler flow velocimetric assessments of placental maturity. Br. J. Obstet. Gynaecol. 1995;102:818–825. - PubMed
-
- Charnock-Jones D. S., Burton G. J. Placental vascular morphogenesis. Baillieres Best Pract. Res. Clin. Obstet. Gynaecol. 2000;14:953–968. - PubMed
-
- Charnock-Jones D. S., Kaufmann P., Mayhew T. M. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004;25:103–113. - PubMed
-
- Chen J., Kuei C., Sutton S., Wilson S., Yu J., Kamme F., Mazur C., Lovenberg T., Liu C. Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1. Mol. Pharmacol. 2005;67:2070–2076. - PubMed
-
- Dellian M., Witwer B. P., Salehi H. A., Yuan F., Jain R. K. Quantitation and physiological characterization of angiogenic vessels in mice: effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am. J. Pathol. 1996;149:59–71. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

