Drug-dependent clearance of human platelets in the NOD/scid mouse by antibodies from patients with drug-induced immune thrombocytopenia
- PMID: 20587782
- PMCID: PMC2974608
- DOI: 10.1182/blood-2010-03-277764
Drug-dependent clearance of human platelets in the NOD/scid mouse by antibodies from patients with drug-induced immune thrombocytopenia
Abstract
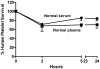
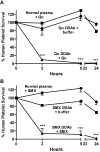
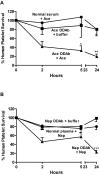
Drug-induced immune thrombocytopenia (DITP) is a relatively common and sometimes life-threatening condition caused by antibodies that bind avidly to platelets only when drug is present. How drug-dependent antibodies (DDAbs) are induced and how drugs promote their interaction with platelets are poorly understood, and methods for detecting DDAbs are suboptimal. A small animal model of DITP could provide a new tool for addressing these and other questions concerning pathogenesis and diagnosis. We examined whether the nonobese diabetic/severe combined immunodeficient (NOD/scid) mouse, which lacks xenoantibodies and therefore allows infused human platelets to circulate, can be used to study drug-dependent clearance of platelets by DDAbs in vivo. In this report, we show that the NOD/scid model is suitable for this purpose and describe studies to optimize its sensitivity for drug-dependent human antibody detection. We further show that the mouse can produce metabolites of acetaminophen and naproxen for which certain drug-dependent antibodies are specific in quantities sufficient to enable these antibodies to cause platelet destruction. The findings indicate that the NOD/scid mouse can provide a unique tool for studying DITP pathogenesis and may be particularly valuable for identifying metabolite-specific antibodies capable of causing immune thrombocytopenia or hemolytic anemia.
Figures



References
-
- Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357(6):580–587. - PubMed
-
- Swisher KK, Li X, Vesely SK, George JN. Drug-induced thrombocytopenia: an updated systematic review, 2008. Drug Saf. 2009;32(1):85–86. - PubMed
-
- George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med. 1998;129(11):886–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

