Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus
- PMID: 20587998
- PMCID: PMC3219502
- DOI: 10.1159/000317134
Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus
Abstract
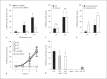
Mechanisms underlying the enhanced virulence phenotype of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) are incompletely defined, but presumably include evasion of killing by human polymorphonuclear leukocytes (PMNs or neutrophils). To better understand this phenomenon, we investigated the basis of rapid PMN lysis after phagocytosis of USA300, a prominent CA-MRSA strain. Survival of USA300 clinical isolates after phagocytosis ultimately resulted in neutrophil lysis. PMNs containing ingested USA300 underwent morphological changes consistent with apoptosis, but lysed rapidly thereafter (within 6 h), whereas cells undergoing FAS-mediated apoptosis or phagocytosis-induced cell death remained intact. Phagosome membranes remained intact until the point of PMN destruction, suggesting lysis was not caused by escape of S. aureus from phagosomes or the cytolytic action of pore-forming toxins. Microarray analysis of the PMN transcriptome after phagocytosis of representative community-associated S. aureus and healthcare-associated MRSA strains revealed changes unique to community-associated S. aureus strains, such as upregulation of transcripts involved in regulation of calcium homeostasis. Collectively, the data suggest that neutrophil destruction after phagocytosis of USA300 is in part a form of programmed necrosis rather than direct lysis by S. aureus pore-forming toxins. We propose that the ability of CA-MRSA strains to induce programmed necrosis of neutrophils is a component of enhanced virulence.
Copyright © 2010 S. Karger AG, Basel.
Figures








References
-
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. - PubMed
-
- Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

