Quantitative fluorescence tomography using a combined tri-modality FT/DOT/XCT system
- PMID: 20588625
- PMCID: PMC2898749
- DOI: 10.1364/OE.18.007835
Quantitative fluorescence tomography using a combined tri-modality FT/DOT/XCT system
Abstract
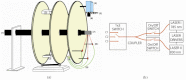
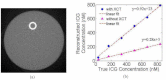
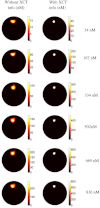
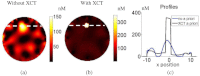
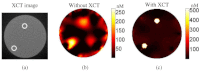
In this work, a first-of-its-kind fully integrated tri-modality system that combines fluorescence, diffuse optical and x-ray tomography (FT/DOT/XCT) into the same setting is presented. The purpose of this system is to perform quantitative fluorescence tomography using multi-modality imaging approach. XCT anatomical information is used as structural priori while optical background heterogeneity information obtained by DOT measurements is used as functional priori. The performance of the hybrid system is evaluated using multi-modality phantoms. In particular, we show that a 2.4 mm diameter fluorescence inclusion located in a heterogeneous medium can be localized accurately with the functional a priori information, although the fluorophore concentration is recovered with 70% error. On the other hand, the fluorophore concentration can be accurately recovered within 8% error only when both DOT optical background functional and XCT structural a priori information are utilized to guide and constrain the FT reconstruction algorithm.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

