Different corticostriatal integration in spiny projection neurons from direct and indirect pathways
- PMID: 20589098
- PMCID: PMC2893005
- DOI: 10.3389/fnsys.2010.00015
Different corticostriatal integration in spiny projection neurons from direct and indirect pathways
Abstract
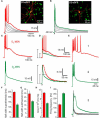
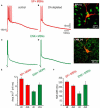
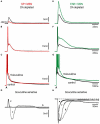
The striatum is the principal input structure of the basal ganglia. Major glutamatergic afferents to the striatum come from the cerebral cortex and make monosynaptic contacts with medium spiny projection neurons (MSNs) and interneurons. Also: glutamatergic afferents to the striatum come from the thalamus. Despite differences in axonal projections, dopamine (DA) receptors expression and differences in excitability between MSNs from "direct" and "indirect" basal ganglia pathways, these neuronal classes have been thought as electrophysiologically very similar. Based on work with bacterial artificial chromosome (BAC) transgenic mice, here it is shown that corticostriatal responses in D(1)- and D(2)-receptor expressing MSNs (D(1)- and D(2)-MSNs) are radically different so as to establish an electrophysiological footprint that readily differentiates between them. Experiments in BAC mice allowed us to predict, with high probability (P > 0.9), in rats or non-BAC mice, whether a recorded neuron, from rat or mouse, was going to be substance P or enkephalin (ENK) immunoreactive. Responses are more prolonged and evoke more action potentials in D(1)-MSNs, while they are briefer and exhibit intrinsic autoregenerative responses in D(2)-MSNs. A main cause for these differences was the interaction of intrinsic properties with the inhibitory contribution in each response. Inhibition always depressed corticostriatal depolarization in D(2)-MSNs, while it helped in sustaining prolonged depolarizations in D(1)-MSNs, in spite of depressing early discharge. Corticostriatal responses changed dramatically after striatal DA depletion in 6-hydroxy-dopamine (6-OHDA) lesioned animals: a response reduction was seen in substance P (SP)+ MSNs whereas an enhanced response was seen in ENK+ MSNs. The end result was that differences in the responses were greatly diminished after DA depletion.
Keywords: 6-hydroxy-dopamine; GABAA receptors; Parkinson disease; basal ganglia; corticostriatal pathway; medium spiny neurons; strionigral pathway; striopallidal pathway.
Figures




Similar articles
-
Dopaminergic modulation of corticostriatal responses in medium spiny projection neurons from direct and indirect pathways.Front Syst Neurosci. 2011 Mar 29;5:15. doi: 10.3389/fnsys.2011.00015. eCollection 2011. Front Syst Neurosci. 2011. PMID: 21483724 Free PMC article.
-
Convergence of cortical and thalamic input to direct and indirect pathway medium spiny neurons in the striatum.Brain Struct Funct. 2014 Sep;219(5):1787-800. doi: 10.1007/s00429-013-0601-z. Epub 2013 Jul 6. Brain Struct Funct. 2014. PMID: 23832596 Free PMC article.
-
Cortical and thalamic innervation of direct and indirect pathway medium-sized spiny neurons in mouse striatum.J Neurosci. 2010 Nov 3;30(44):14610-8. doi: 10.1523/JNEUROSCI.1623-10.2010. J Neurosci. 2010. PMID: 21048118 Free PMC article.
-
D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons.Trends Neurosci. 2007 May;30(5):228-35. doi: 10.1016/j.tins.2007.03.008. Epub 2007 Apr 3. Trends Neurosci. 2007. PMID: 17408758 Review.
-
Sex Differences in Medium Spiny Neuron Excitability and Glutamatergic Synaptic Input: Heterogeneity Across Striatal Regions and Evidence for Estradiol-Dependent Sexual Differentiation.Front Endocrinol (Lausanne). 2018 Apr 18;9:173. doi: 10.3389/fendo.2018.00173. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29720962 Free PMC article. Review.
Cited by
-
Dopamine imbalance in Huntington's disease: a mechanism for the lack of behavioral flexibility.Front Neurosci. 2013 Jul 4;7:114. doi: 10.3389/fnins.2013.00114. eCollection 2013. Front Neurosci. 2013. PMID: 23847463 Free PMC article.
-
Temporal convergence of dynamic cell assemblies in the striato-pallidal network.J Neurosci. 2012 Feb 15;32(7):2473-84. doi: 10.1523/JNEUROSCI.4830-11.2012. J Neurosci. 2012. PMID: 22396421 Free PMC article.
-
Diverse Short-Term Dynamics of Inhibitory Synapses Converging on Striatal Projection Neurons: Differential Changes in a Rodent Model of Parkinson's Disease.Neural Plast. 2015;2015:573543. doi: 10.1155/2015/573543. Epub 2015 Jun 8. Neural Plast. 2015. PMID: 26167304 Free PMC article.
-
Duration differences of corticostriatal responses in striatal projection neurons depend on calcium activated potassium currents.Front Syst Neurosci. 2013 Oct 4;7:63. doi: 10.3389/fnsys.2013.00063. eCollection 2013. Front Syst Neurosci. 2013. PMID: 24109439 Free PMC article.
-
An Improved BAC Transgenic Fluorescent Reporter Line for Sensitive and Specific Identification of Striatonigral Medium Spiny Neurons.Front Syst Neurosci. 2011 Jun 8;5:32. doi: 10.3389/fnsys.2011.00032. eCollection 2011. Front Syst Neurosci. 2011. PMID: 21713123 Free PMC article.
References
LinkOut - more resources
Full Text Sources

