Nitric oxide enhances inhibitory synaptic transmission and neuronal excitability in Guinea-pig submucous plexus
- PMID: 20589236
- PMCID: PMC2904599
- DOI: 10.3389/fnins.2010.00030
Nitric oxide enhances inhibitory synaptic transmission and neuronal excitability in Guinea-pig submucous plexus
Abstract
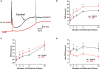
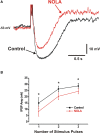
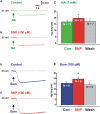
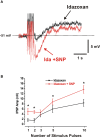
Varicosities immunoreactive for nitric oxide synthase (NOS) make synaptic connections with submucosal neurons in the guinea-pig small intestine, but the effects of nitric oxide (NO) on these neurons are unknown. We used intracellular recording to characterize effects of sodium nitroprusside (SNP, NO donor) and nitro-l-arginine (NOLA, NOS inhibitor), on inhibitory synaptic potentials (IPSPs), slow excitatory synaptic potentials (EPSPs) and action potential firing in submucosal neurons of guinea-pig ileum in vitro. Recordings were made from neurons with the characteristic IPSPs of non-cholinergic secretomotor neurons. SNP (100 muM) markedly enhanced IPSPs evoked by single stimuli applied to intermodal strands and IPSPs evoked by trains of 2-10 pulses (30 Hz). Both noradrenergic (idazoxan-sensitive) and non-adrenergic (idazoxan-insensitive) IPSPs were affected. SNP enhanced hyperpolarizations evoked by locally applied noradrenaline or somatostatin. SNP did not affect slow EPSPs evoked by single stimuli, but depressed slow EPSPs evoked by stimulus trains. NOLA (100 muM) depressed IPSPs evoked by one to three stimulus pulses and enhanced slow EPSPs evoked by trains of two to three stimuli (30 Hz). SNP also increased the number of action potentials and the duration of firing evoked by prolonged (500 or 1000 ms) depolarizing current pulses, but NOLA had no consistent effect on action potential firing. We conclude that neurally released NO acts post-synaptically to enhance IPSPs and depress slow EPSPs, but may enhance the intrinsic excitability of these neurons. Thus, NOS neurons may locally regulate several secretomotor pathways ending on common neurons.
Figures

 denotes an inhibitory synapse.
denotes an inhibitory synapse.References
LinkOut - more resources
Full Text Sources

