A phase I study evaluating the role of the anti-epidermal growth factor receptor (EGFR) antibody cetuximab as a radiosensitizer with chemoradiation for locally advanced pancreatic cancer
- PMID: 20589377
- PMCID: PMC3434707
- DOI: 10.1007/s00280-010-1383-0
A phase I study evaluating the role of the anti-epidermal growth factor receptor (EGFR) antibody cetuximab as a radiosensitizer with chemoradiation for locally advanced pancreatic cancer
Abstract
Purpose: (1) To determine the safety of the epidermal growth factor receptor (EGFR) antibody cetuximab with concurrent gemcitabine and abdominal radiation in the treatment of patients with locally advanced adenocarcinoma of the pancreas. (2) To evaluate the feasibility of pancreatic cancer cell epithelial-mesenchymal transition (EMT) molecular profiling as a potential predictor of response to anti-EGFR treatment.
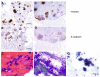
Methods: Patients with non-metastatic, locally advanced pancreatic cancer were treated in this dose escalation study with gemcitabine (0-300 mg/m(2)/week) given concurrently with cetuximab (400 mg/m(2) loading dose, 250 mg/m(2) weekly maintenance dose) and abdominal irradiation (50.4 Gy). Expression of E-cadherin and vimentin was assessed by immunohistochemistry in diagnostic endoscopic ultrasound fine-needle aspiration (EUS-FNA) specimens.
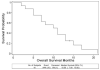
Results: Sixteen patients were enrolled in 4 treatment cohorts with escalating doses of gemcitabine. Incidence of grade 1-2 adverse events was 96%, and incidence of 3-4 adverse events was 9%. There were no treatment-related mortalities. Two patients who exhibited favorable treatment response underwent surgical exploration and were intraoperatively confirmed to have unresectable tumors. Median overall survival was 10.5 months. Pancreatic cancer cell expression of E-cadherin and vimentin was successfully determined in EUS-FNA specimens from 4 patients.
Conclusions: Cetuximab can be safely administered with abdominal radiation and concurrent gemcitabine (up to 300 mg/m(2)/week) in patients with locally advanced adenocarcinoma of the pancreas. This combined therapy modality exhibited limited activity. Diagnostic EUS-FNA specimens could be analyzed for molecular markers of EMT in a minority of patients with pancreatic cancer.
Figures
Similar articles
-
Randomized phase II--study evaluating EGFR targeting therapy with cetuximab in combination with radiotherapy and chemotherapy for patients with locally advanced pancreatic cancer--PARC: study protocol [ISRCTN56652283].BMC Cancer. 2005 Oct 11;5:131. doi: 10.1186/1471-2407-5-131. BMC Cancer. 2005. PMID: 16219105 Free PMC article. Clinical Trial.
-
Neoadjuvant cetuximab, twice-weekly gemcitabine, and intensity-modulated radiotherapy (IMRT) in patients with pancreatic adenocarcinoma.Ann Oncol. 2012 Nov;23(11):2820-2827. doi: 10.1093/annonc/mds109. Epub 2012 May 9. Ann Oncol. 2012. PMID: 22571859 Free PMC article. Clinical Trial.
-
Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial.J Clin Oncol. 2004 Jul 1;22(13):2610-6. doi: 10.1200/JCO.2004.12.040. J Clin Oncol. 2004. PMID: 15226328 Clinical Trial.
-
New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways.Oncologist. 2008 Mar;13(3):289-98. doi: 10.1634/theoncologist.2007-0134. Oncologist. 2008. PMID: 18378539 Review.
-
Epidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancers.Semin Oncol. 2002 Feb;29(1 Suppl 4):27-36. doi: 10.1053/sonc.2002.31525. Semin Oncol. 2002. PMID: 11894011 Review.
Cited by
-
Prospective study of cetuximab and gemcitabine in combination with radiation therapy: feasibility and efficacy in locally advanced pancreatic head cancer.Radiat Oncol. 2015 Dec 15;10:255. doi: 10.1186/s13014-015-0564-8. Radiat Oncol. 2015. PMID: 26670587 Free PMC article. Clinical Trial.
-
Arterial complication of irreversible electroporation procedure for locally advanced pancreatic cancer.World J Gastrointest Oncol. 2016 Oct 15;8(10):751-756. doi: 10.4251/wjgo.v8.i10.751. World J Gastrointest Oncol. 2016. PMID: 27795815 Free PMC article.
-
CT Findings of Patients Treated with Irreversible Electroporation for Locally Advanced Pancreatic Cancer.J Oncol. 2015;2015:680319. doi: 10.1155/2015/680319. Epub 2015 Nov 5. J Oncol. 2015. PMID: 26649039 Free PMC article.
-
Targeting ErbB3-mediated stromal-epithelial interactions in pancreatic ductal adenocarcinoma.Br J Cancer. 2011 Aug 9;105(4):523-33. doi: 10.1038/bjc.2011.263. Epub 2011 Jul 26. Br J Cancer. 2011. PMID: 21792199 Free PMC article.
-
Phase II Trial of Cetuximab and Conformal Radiotherapy Only in Locally Advanced Pancreatic Cancer with Concurrent Tissue Sampling Feasibility Study.Transl Oncol. 2014 Feb 1;7(1):55-64. doi: 10.1593/tlo.13724. eCollection 2014 Feb. Transl Oncol. 2014. PMID: 24772208 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Evans DB, Rich TA, Byrd DR, et al. Adenocarcinoma of the pancreas: current management of resectable and locally advanced disease. South Med J. 1991;84:566–570. - PubMed
-
- Willett CG, Czito BG, Bendell JC, et al. Locally advanced pancreatic cancer. J Clin Oncol. 2005;23:4538–4544. - PubMed
-
- Coia L, Hoffman J, Scher R, et al. Preoperative chemoradiation for adenocarcinoma of the pancreas and duodenum. Int J Radiat Oncol Biol Phys. 1994;30:161–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

