On the pH-optimum of activity and stability of proteins
- PMID: 20589630
- PMCID: PMC2911520
- DOI: 10.1002/prot.22786
On the pH-optimum of activity and stability of proteins
Abstract
Biological macromolecules evolved to perform their function in specific cellular environment (subcellular compartments or tissues); therefore, they should be adapted to the biophysical characteristics of the corresponding environment, one of them being the characteristic pH. Many macromolecular properties are pH dependent, such as activity and stability. However, only activity is biologically important, while stability may not be crucial for the corresponding reaction. Here, we show that the pH-optimum of activity (the pH of maximal activity) is correlated with the pH-optimum of stability (the pH of maximal stability) on a set of 310 proteins with available experimental data. We speculate that such a correlation is needed to allow the corresponding macromolecules to tolerate small pH fluctuations that are inevitable with cellular function. Our findings rationalize the efforts of correlating the pH of maximal stability and the characteristic pH of subcellular compartments, as only pH of activity is subject of evolutionary pressure. In addition, our analysis confirmed the previous observation that pH-optimum of activity and stability are not correlated with the isoelectric point, pI, or with the optimal temperature.
Copyright 2010 Wiley-Liss, Inc.
Figures

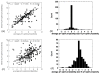
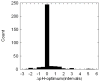
Similar articles
-
Subcellular pH and predicted pH-dependent features of proteins.Proteomics. 2006 Jun;6(12):3494-501. doi: 10.1002/pmic.200500534. Proteomics. 2006. PMID: 16705750
-
Evidence for the adaptation of protein pH-dependence to subcellular pH.BMC Biol. 2009 Oct 22;7:69. doi: 10.1186/1741-7007-7-69. BMC Biol. 2009. PMID: 19849832 Free PMC article.
-
Numerical calculations of the pH of maximal protein stability. The effect of the sequence composition and three-dimensional structure.Eur J Biochem. 2004 Jan;271(1):173-85. doi: 10.1046/j.1432-1033.2003.03917.x. Eur J Biochem. 2004. PMID: 14686930
-
On the role of electrostatics in protein-protein interactions.Phys Biol. 2011 Jun;8(3):035001. doi: 10.1088/1478-3975/8/3/035001. Epub 2011 May 13. Phys Biol. 2011. PMID: 21572182 Free PMC article. Review.
-
Stabilization of proteins for storage.Cold Spring Harb Protoc. 2010 May;2010(5):pdb.top79. doi: 10.1101/pdb.top79. Cold Spring Harb Protoc. 2010. PMID: 20439424 Review.
Cited by
-
Structural restrictions for influenza neuraminidase activity promote adaptation and diversification.Nat Microbiol. 2019 Dec;4(12):2565-2577. doi: 10.1038/s41564-019-0537-z. Epub 2019 Aug 26. Nat Microbiol. 2019. PMID: 31451775
-
Computational investigation of proton transfer, pKa shifts and pH-optimum of protein-DNA and protein-RNA complexes.Proteins. 2017 Feb;85(2):282-295. doi: 10.1002/prot.25221. Epub 2017 Jan 5. Proteins. 2017. PMID: 27936518 Free PMC article.
-
Mitochondrial carbonic anhydrase VA and VB: properties and roles in health and disease.J Physiol. 2023 Jan;601(2):257-274. doi: 10.1113/JP283579. Epub 2022 Dec 19. J Physiol. 2023. PMID: 36464834 Free PMC article. Review.
-
The Molecular Effects of Dietary Acid Load on Metabolic Disease (The Cellular PasaDoble: The Fast-Paced Dance of pH Regulation).Front Mol Med. 2021 Nov 16;1:777088. doi: 10.3389/fmmed.2021.777088. eCollection 2021. Front Mol Med. 2021. PMID: 39087082 Free PMC article. Review.
-
A Newton-like iterative method implemented in the DelPhi for solving the nonlinear Poisson-Boltzmann equation.Math Biosci Eng. 2020 Sep 21;17(6):6259-6277. doi: 10.3934/mbe.2020331. Math Biosci Eng. 2020. PMID: 33378855 Free PMC article.
References
-
- Horiguchi M, Arita M, Kaempf-Rotzoll DE, Tsujimoto M, Inoue K, Arai H. pH-dependent translocation of alpha-tocopherol transfer protein (alpha-TTP) between hepatic cytosol and late endosomes. Genes Cells. 2003;8(10):789–800. - PubMed
-
- Imamoto Y, Harigai M, Kataoka M. Direct observation of the pH-dependent equilibrium between L-like and M intermediates of photoactive yellow protein. FEBS Lett. 2004;577(1–2):75–80. - PubMed
-
- Grey MJ, Tang Y, Alexov E, McKnight CJ, Raleigh DP, Palmer AG., 3rd Characterizing a partially folded intermediate of the villin headpiece domain under non-denaturing conditions: contribution of His41 to the pH-dependent stability of the N-terminal subdomain. J Mol Biol. 2006;355(5):1078–1094. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

