Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls
- PMID: 20590555
- PMCID: PMC2931546
- DOI: 10.1111/j.1476-5381.2010.00787.x
Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls
Abstract
Endocannabinoids play an important role in a diverse range of neurophysiological processes including neural development, neuroimmune function, synaptic plasticity, pain, reward and affective state. This breadth of influence and evidence for altered endocannabinoid signalling in a variety of neuropathologies has fuelled interest in the accurate quantification of these lipids in brain tissue. Established methods for endocannabinoid quantification primarily employ solvent-based lipid extraction with further sample purification by solid phase extraction. In recent years in vivo microdialysis methods have also been developed for endocannabinoid sampling from the brain interstitial space. However, considerable variability in estimates of endocannabinoid content has led to debate regarding the physiological range of concentrations present in various brain regions. This paper provides a critical review of factors that influence the quantification of brain endocannabinoid content as determined by lipid extraction from bulk tissue and by in vivo microdialysis. A variety of methodological issues are discussed including analytical approaches, endocannabinoid extraction and purification, post-mortem changes in brain endocannabinoid content, cellular reactions to microdialysis probe implantation and caveats related to lipid sampling from the extracellular space. The application of these methods for estimating brain endocannabinoid content and the effects of endocannabinoid clearance inhibition are discussed. The benefits, limitations and pitfalls associated with each approach are emphasized, with an eye toward the appropriate interpretation of data gathered by each method.
Figures

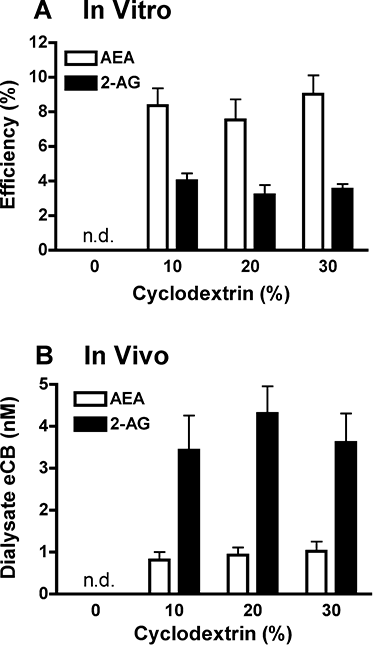
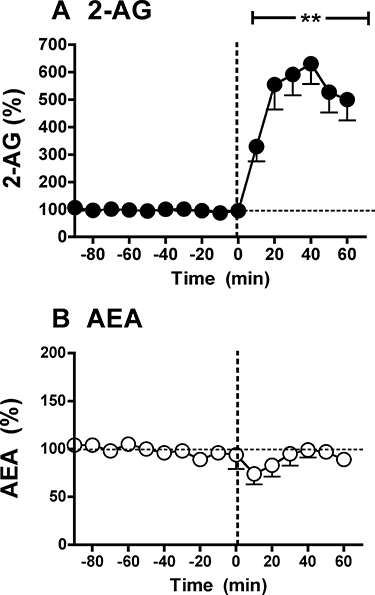
References
-
- Abulrob A, Tauskela JS, Mealing G, Brunette E, Faid K, Stanimirovic D. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-D-aspartate receptor redistribution. J Neurochem. 2005;92:1477–1486. - PubMed
-
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

