Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery?
- PMID: 20590557
- PMCID: PMC2931548
- DOI: 10.1111/j.1476-5381.2010.00777.x
Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery?
Abstract
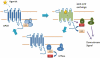
The main pharmacological effects of marijuana, as well as synthetic and endogenous cannabinoids, are mediated through G-protein-coupled receptors (GPCRs), including CB(1) and CB(2) receptors. The CB(1) receptor is the major cannabinoid receptor in the central nervous system and has gained increasing interest as a target for drug discovery for treatment of nausea, cachexia, obesity, pain, spasticity, neurodegenerative diseases and mood and substance abuse disorders. Evidence has accumulated to suggest that CB(1) receptors, like other GPCRs, interact with and are regulated by several other proteins beyond the established role of heterotrimeric G-proteins. These proteins, which include the GPCR kinases, beta-arrestins, GPCR-associated sorting proteins, factor associated with neutral sphingomyelinase, other GPCRs (heterodimerization) and the novel cannabinoid receptor-interacting proteins: CRIP(1a/b), are thought to play important roles in the regulation of intracellular trafficking, desensitization, down-regulation, signal transduction and constitutive activity of CB(1) receptors. This review examines CB(1) receptor-interacting proteins, including heterotrimeric G-proteins, but with particular emphasis on non-G-protein entities, that might comprise the CB(1) receptosomal complex. The evidence for direct interaction with CB(1) receptors and potential functional roles of these interacting proteins is discussed, as are future directions and challenges in this field with an emphasis on the possibility of eventually targeting these proteins for drug discovery.
Figures


References
-
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, et al. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. - PubMed
-
- Baldwin JM, Schertler GF, Unger VM. An alpha-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J Mol Biol. 1997;272:144–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

